- Record: found
- Abstract: found
- Article: found
GOLPH3 Is Essential for Contractile Ring Formation and Rab11 Localization to the Cleavage Site during Cytokinesis in Drosophila melanogaster
Read this article at
Abstract
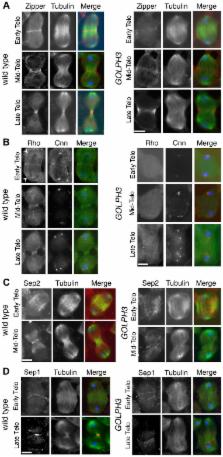
The highly conserved Golgi phosphoprotein 3 (GOLPH3) protein has been described as a Phosphatidylinositol 4-phosphate [PI(4)P] effector at the Golgi. GOLPH3 is also known as a potent oncogene, commonly amplified in several human tumors. However, the molecular pathways through which the oncoprotein GOLPH3 acts in malignant transformation are largely unknown. GOLPH3 has never been involved in cytokinesis. Here, we characterize the Drosophila melanogaster homologue of human GOLPH3 during cell division. We show that GOLPH3 accumulates at the cleavage furrow and is required for successful cytokinesis in Drosophila spermatocytes and larval neuroblasts. In premeiotic spermatocytes GOLPH3 protein is required for maintaining the organization of Golgi stacks. In dividing spermatocytes GOLPH3 is essential for both contractile ring and central spindle formation during cytokinesis. Wild type function of GOLPH3 enables maintenance of centralspindlin and Rho1 at cell equator and stabilization of Myosin II and Septin rings. We demonstrate that the molecular mechanism underlying GOLPH3 function in cytokinesis is strictly dependent on the ability of this protein to interact with PI(4)P. Mutations that abolish PI(4)P binding impair recruitment of GOLPH3 to both the Golgi and the cleavage furrow. Moreover telophase cells from mutants with defective GOLPH3-PI(4)P interaction fail to accumulate PI(4)P-and Rab11-associated secretory organelles at the cleavage site. Finally, we show that GOLPH3 protein interacts with components of both cytokinesis and membrane trafficking machineries in Drosophila cells. Based on these results we propose that GOLPH3 acts as a key molecule to coordinate phosphoinositide signaling with actomyosin dynamics and vesicle trafficking during cytokinesis. Because cytokinesis failures have been associated with premalignant disease and cancer, our studies suggest novel insight into molecular circuits involving the oncogene GOLPH3 in cytokinesis.
Author Summary
In animal cell cytokinesis, constriction of an actomyosin ring at the equatorial cortex of dividing cells must be finely coordinated with plasma membrane remodeling and vesicle trafficking at the cleavage furrow. Accurate control of these events during cell cleavage is essential for maintaining ploidy and preventing neoplastic transformation. GOLPH3 has been recognized as a potent oncogene, involved in the development of several human tumors. However, the precise roles played by GOLPH3 in tumorigenesis are not yet understood. In this manuscript we demonstrate for the first time the requirement for GOLPH3 for cytokinesis. GOLPH3 protein localizes at the cleavage site of Drosophila dividing cells and is essential for cytokinesis in male meiotic cells and larval neuroblasts. We show that this protein acts as a key molecule in coupling plasma membrane remodeling with actomyosin ring assembly and stability during cytokinesis. Our studies indicate a novel connection between GOLPH3 and the molecular mechanisms of cytokinesis, opening new fields of investigation into the tumor cell biology of this oncogene.
Related collections
Most cited references60
- Record: found
- Abstract: found
- Article: not found
Cytokinesis in animal cells.
- Record: found
- Abstract: found
- Article: not found
A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell.
- Record: found
- Abstract: found
- Article: not found