- Record: found
- Abstract: found
- Article: found
Non‐Thermal Plasma as a Unique Delivery System of Short‐Lived Reactive Oxygen and Nitrogen Species for Immunogenic Cell Death in Melanoma Cells

Read this article at
Abstract
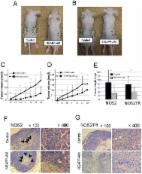
Breakthroughs in cancer immunotherapies have demonstrated considerable success, though not without limitations. Non‐thermal plasma (NTP) for cancer therapy has been emerging as a potential adjuvant treatment via induction of immunogenic cell death (ICD). Cancer cells undergoing ICD stimulate a patient's immune system to mount an anticancer response. While promising, the underlying mechanisms of NTP‐induced ICD must be closely examined. Here, the interaction between non‐thermal plasma and cancerous cells is studied. The short‐lived reactive oxygen and nitrogen species (e.g., hydroxyl radicals, atomic oxygen, nitric oxide) produced by plasma are the main effectors that elicit ICD in melanoma while, surprisingly, persistent species do not. This is demonstrated in vitro using a dielectric barrier discharge plasma system and is validated in a vaccination assay in vivo. Plasma generation of reactive species appears to be dictated by the total energy. Collectively, this work provides fundamental insight into plasma interactions with biological material. Furthermore, it lays the foundation for future development of NTP systems for clinical translation. The addition of plasma systems into the existing arsenal of cancer therapies opens the possibility for new combination strategies for safer and more robust control of cancer.
Related collections
Most cited references9
- Record: found
- Abstract: found
- Article: not found
ROS implication in a new antitumor strategy based on non-thermal plasma.
- Record: found
- Abstract: not found
- Article: not found
On atmospheric-pressure non-equilibrium plasma jets and plasma bullets
- Record: found
- Abstract: found
- Article: found