- Record: found
- Abstract: found
- Article: found
Cellular Stresses and Stress Responses in the Pathogenesis of Insulin Resistance
Read this article at
Abstract
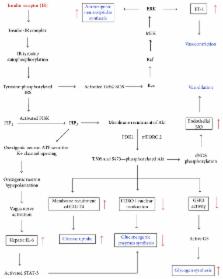
Insulin resistance (IR), a key component of the metabolic syndrome, precedes the development of diabetes, cardiovascular disease, and Alzheimer's disease. Its etiological pathways are not well defined, although many contributory mechanisms have been established. This article summarizes such mechanisms into the hypothesis that factors like nutrient overload, physical inactivity, hypoxia, psychological stress, and environmental pollutants induce a network of cellular stresses, stress responses, and stress response dysregulations that jointly inhibit insulin signaling in insulin target cells including endothelial cells, hepatocytes, myocytes, hypothalamic neurons, and adipocytes. The insulin resistance-inducing cellular stresses include oxidative, nitrosative, carbonyl/electrophilic, genotoxic, and endoplasmic reticulum stresses; the stress responses include the ubiquitin-proteasome pathway, the DNA damage response, the unfolded protein response, apoptosis, inflammasome activation, and pyroptosis, while the dysregulated responses include the heat shock response, autophagy, and nuclear factor erythroid-2-related factor 2 signaling. Insulin target cells also produce metabolites that exacerbate cellular stress generation both locally and systemically, partly through recruitment and activation of myeloid cells which sustain a state of chronic inflammation. Thus, insulin resistance may be prevented or attenuated by multiple approaches targeting the different cellular stresses and stress responses.
Related collections
Most cited references321
- Record: found
- Abstract: found
- Article: not found
ER stress-induced cell death mechanisms.
- Record: found
- Abstract: found
- Article: not found
NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice.
- Record: found
- Abstract: found
- Article: not found