- Record: found
- Abstract: found
- Article: not found
A fifth amendment to the intestine's constitution
news
Publication date (Print):
7 March 2011
Journal:
The Journal of Cell Biology
Publisher:
The Rockefeller University Press
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
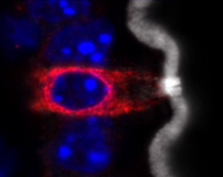
A tuft cell (red) with a thick brush of microvilli (white) in the epithelium of an
intestinal villus.
Gerbe et al. define a new type of secretory cell in the intestine.
The intestinal epithelium consists of four main specialized cell lineages: absorptive
enterocytes and three secretory cell types known as enteroendocrine, Paneth, and goblet
cells. But a rare, fifth type of intestinal cell called tuft cells also exists. Defined
by the thick brush of long microvilli that project from their apical surface, tuft
cells are seen in several epithelial tissues, yet little is known about their function
due to a lack of tuft cell–specific markers.
Gerbe et al. identified several proteins uniquely expressed by tuft cells, including
DCLK1, a kinase that was previously thought to mark a population of quiescent intestinal
stem cells. Like other intestinal cell types, tuft cells turned over rapidly and were
replaced by the differentiation of proliferative stem cells' progeny in the intestinal
crypts. This differentiation was blocked in the absence of ATOH1—a transcription factor
required for the development of all intestinal secretory lineages. Yet tuft cell differentiation
didn't require other transcription factors that specify enteroendocrine, Paneth, and
goblet cells, suggesting that tuft cells represent a distinct lineage of intestinal
secretory cells.
Gerbe et al. found that tuft cells secrete opioids and produce enzymes that synthesize
prostaglandins. The latter observation suggests that tuft cells may promote inflammation
and tumorigenesis. Indeed, the researchers identified tuft cell–like cells in several
early stage intestinal tumors. To really understand tuft cells' function, however,
author Philippe Jay hopes to identify transcription factors uniquely required for
their development in order to generate mice that specifically lack tuft cells from
their intestinal epithelium.
Related collections
Most cited references1

- Record: found
- Abstract: found
- Article: found
Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium
François Gerbe, Johan H van Es, Leila Makrini … (2011)
Introduction The intestinal epithelium is a highly dynamic tissue with continuous proliferation, migration, differentiation, and apoptosis, resulting in complete renewal every 2–7 d, in a spatially and temporally organized manner. This process is coordinated by a small number of highly conserved signaling pathways (Sancho et al., 2004). While migrating toward the villi, progenitor cells differentiate into distinct cell types that can be identified using morphological criteria and through expression of specific genes. Differentiated epithelial cells belong to two classes: absorptive enterocytes and secretory cells. Secretory cells can be further subdivided into three cell types: mucus-producing goblet cells, hormone-secreting enteroendocrine cells, and bactericidal Paneth cells. The composition of the villus epithelium mainly results from the interaction of signaling pathways that are active in crypt stem and progenitor cells. The most studied examples are the Wingless-related MMTV integration site (Wnt) and Notch pathways. Inhibition of the Wnt signaling pathway induces a complete loss of crypt epithelial progenitors (Korinek et al., 1998; Pinto et al., 2003). Genetic and pharmacologic inhibition of the Notch pathway drives the cells toward a secretory fate, even though the Wnt cascade remains active (Fre et al., 2005; van Es et al., 2005b) and, accordingly, deletion of the Notch effector hairy/enhancer of split 1 (Hes1) results in the generation of excessive numbers of goblet, enteroendocrine, and Paneth cells (Jensen et al., 2000; Suzuki et al., 2005). Conversely, the basic-helix-loop-helix transcription factor encoded by the Atonal homologue 1 gene (Atoh1, also called Math1), which is repressed by the HES1 transcription factor, is required for a progenitor cell to adopt a secretory fate (Yang et al., 2001; Shroyer et al., 2007; van Es et al., 2010). It is often thought that a single Atoh1-dependent secretory progenitor exists for all three secretory cell types. However, some data instead point toward the existence of several bi-potential progenitors that can each produce either an enterocyte or a secretory cell belonging to the goblet, enteroendocrine, or Paneth cell type, a fate choice that likely relies on Notch signaling (Bjerknes and Cheng, 1999). In addition to ATOH1, a set of transcription factors determines the cell fate choice and differentiation toward goblet, Paneth, or enteroendocrine cell types. Neurogenin 3 (Neurog3, also called Ngn3) is essential for all intestinal enteroendocrine cells (Jenny et al., 2002; Mellitzer et al., 2010) and has been reported to be repressed by the growth factor-independent 1 (GFI1) transcription factor, which is normally expressed in both Paneth and goblet cells (Bjerknes and Cheng, 2010). Deletion of the Gfi1 gene, in turn, results in an increased enteroendocrine cell population at the expense of Paneth and goblet cells (Shroyer et al., 2005), likely due to cellular reprogramming of Paneth and goblet cells toward a Neurog3+ enteroendocrine cell phenotype (Bjerknes and Cheng, 2010). The Kruppel-like factor 4 (Klf4) and SAM pointed domain containing Ets transcription factor (Spdef) genes are required for terminal differentiation of goblet cells (Katz et al., 2002). Spdef is necessary for Paneth cell maturation (Gregorieff et al., 2009), and differentiation is shifted toward the goblet cell type, at the expense of the absorptive as well as Paneth and enteroendocrine cell types in the intestinal epithelium of transgenic animals overexpressing Spdef (Noah et al., 2010). Finally, the SRY-box containing gene 9 (Sox9) is essential for differentiation of Paneth cells (Bastide et al., 2007; Mori-Akiyama et al., 2007), and Wnt signaling through the Frizzled-5 receptor is required for their terminal maturation (van Es et al., 2005a). The permanent turnover of the intestinal epithelium relies on the self-renewing capacity of stem cells. The Wnt target gene Leucine-rich repeat containing G protein–coupled receptor 5 (Lgr5) has been identified as a marker of crypt base columnar (CBC) cells (Barker et al., 2007). Genetic lineage-tracing experiments revealed that CBC cells are multipotent and self-renewing, thus representing genuine intestinal stem cells (Bjerknes and Cheng, 1999; Barker et al., 2007). In addition, cells located above the Paneth cell compartment (also known as the +4 position) and expressing the Bmi1 polycomb ring finger oncogene (Bmi1) have been reported to have features of stem cells (Sangiorgi and Capecchi, 2008). However, the presence of these cells is limited to the duodenum, and recent studies showed that Bmi1 expression is, at least partially, overlapping with Lgr5 expression (van der Flier et al., 2009). Both Lgr5 + and Bmi1 + cells are actively cycling, and the presence of a long-lived quiescent stem cell population in the intestinal epithelium is still a matter of debate. For instance, we have recently demonstrated that the solitary cells expressing the doublecortin-like kinase 1 protein (DCLK1, also called DCAMKL1), which have been considered as putative quiescent stem cells (Giannakis et al., 2006; May et al., 2008, 2009; Dekaney et al., 2009; Jin et al., 2009; Sureban et al., 2009), are bona fide tuft cells (Gerbe et al., 2009). Since their first identification in the rat trachea (Rhodin and Dalhamn, 1956) and mouse gastrointestinal tract (Jarvi and Keyrilainen, 1956), tuft cells (also known as brush cells) have been found in several endoderm-derived epithelia. These cells are characterized by long and blunt microvilli with prominent rootlets, and by a well-developed tubulovesicular system in the supranuclear cytoplasm (Sato, 2007). Several markers have been proposed for tuft cells, including villin, fimbrin (Höfer and Drenckhahn, 1992), neuronal nitric oxyde synthase (Kugler et al., 1994), α-gustducin (Höfer et al., 1996), Ulex europaeus lectin 1 (Gebhard and Gebert, 1999; Gebert et al., 2000), Cytokeratin 18 (Höfer and Drenckhahn, 1996), and the transient receptor potential cation channel, subfamily M, member 5 (TRPM5; Bezençon et al., 2007). However, due to their ubiquitous expression in the intestinal epithelium, villin and fimbrin are not very suitable markers of intestinal tuft cells (Höfer and Drenckhahn, 1996). Similarly, α-gustducin, Trpm5, and Ulex europaeus lectin 1 expression have also been reported in subtypes of enteroendocrine cells (Jang et al., 2007; Sutherland et al., 2007; Bezençon et al., 2008; Kokrashvili et al., 2009). Finally, the high neuronal nitric oxyde synthase expression reported for stomachal and pancreatic tuft cells (Kugler et al., 1994) is not a property of intestinal tuft cells (Sutherland et al., 2007), and the validity of Cytokeratin 18 as a marker for mouse intestinal tuft cells is controversial (Gebert et al., 2000). Thus, none of the above markers is strictly tuft cell specific and, more than 50 years after their initial discovery, functional studies of tuft cells are still nonexistent. Here, we report a marker signature that allows unambiguous identification of mouse and human tuft cells, both in the small and large intestines. We extend our previous study to demonstrate that DCLK1-expressing tuft cells are short lived, post-mitotic cells that are permanently generated from Lgr5-expressing stem cells. Furthermore, unlike what is commonly thought, we show that tuft cells do not belong to the enteroendocrine lineage, but rather constitute a distinct entity with transcription factor requirements for differentiation that differ from those of enterocytes, enteroendocrine, Paneth, and goblet cells. Results A set of molecular markers allows unambiguous identification of tuft cells in the mouse intestinal epithelium Trpm5-expressing cells, hypothesized to be tuft cells, were previously shown to express the cyclooxygenase 1 and 2 (COX1 and COX2) enzymes (Bezençon et al., 2008), and we recently found that expression of the DCLK1 protein is a specific marker of tuft cells (Gerbe et al., 2009). We have now extended these observations with multiple costaining experiments of the mouse small intestinal epithelium, based on previously published micro-array data (Bezençon et al., 2008). This allows us to identify a unique marker signature of tuft cells, which includes coexpression of SOX9, COX1, COX2, hematopoietic prostaglandin-D synthase (HPGDS), and DCLK1 (Fig. 1, A–C and F). Compared with other epithelial cells, cells displaying this signature show a stronger immunoreactivity toward villin, α-tubulin and F-actin, which is a typical feature of tuft cells (Höfer and Drenckhahn, 1996; Fig. 1, D, E, and G). The SOX9–COX1 or the HPGDS–COX1 limited signature allowed unambiguous identification of tuft cells in the mouse and human colon, as well as in other epithelia such as in the mouse gall bladder (Fig. S1, A–C). Figure 1. Molecular characterization of mouse intestinal tuft cells. Immunofluorescent stainings for (A) SOX9 and COX1, (B) SOX9 and COX2, (C) HPGDS and COX1, (D) villin and COX1, (E) α-tubulin and COX1, and (F) DCLK1 and COX1. Each panel contains a merged image on the left, and gray level pictures of the indicated individual markers corresponding to the yellow inset area on the right. (G) Whole-mount immunofluorescent staining for DCLK1 and F-actin on a dissociated fragment of intestinal epithelium. Panels on the right show higher magnification of the cropped area of the overlay image. Yellow arrowheads point at tuft cells. Nuclei are stained with Hoechst (blue). Bars, 10 µm. All DCLK1+ cells are post mitotic tuft cells Because DCLK1-expressing cells lack differentiation markers typical of the other intestinal epithelial cells, and do not proliferate, DCLK1 has been considered as a marker of putative quiescent intestinal epithelial stem cells (Giannakis et al., 2006; May et al., 2008, 2009; Dekaney et al., 2009; Jin et al., 2009; Sureban et al., 2009). We previously reported, like others (Giannakis et al., 2006; May et al., 2009), that DCLK1+ cells are never observed in a proliferative state. We further confirm this using the proliferating cell nuclear antigen (PCNA; Fig. 2 A), and Ki67 or phospho-histone H3 (Fig. S1 D) as proliferation markers (n > 200 cells). Furthermore, DCLK1-expressing cells express typical tuft cell markers (Gerbe et al., 2009). Yet, the possibility existed that we had overlooked a second, nontuft fraction of DCLK1+ cells, which might represent quiescent stem cells. To clarify this important point we first compared the level of DCLK1 expression with that of other tuft cell markers and found that 98.1% of DCLK1+ cells were COX1+ (n = 253), the rare DCLK1+ COX1− cells being mainly found in the lower half of the crypts. To further characterize these DCLK1+ COX1− cells present in crypt bottoms, we exploited one of the unique morphological features of tuft cells that is not found in other epithelial cells: the axial bundles of actin filaments supporting the microvilli (Höfer and Drenckhahn, 1998), which can be visualized with phalloidin. An intense, apical staining of F-actin with phalloidin was found in 100% of DCLK1+ tuft cells present in crypt bottoms (n = 74). In addition, we found that SOX9 expression is higher in tuft cells present in crypt bottoms than in Paneth or CBC stem cells. This demonstrates that although COX1 is barely detectable in differentiating tuft cells, strong SOX9 expression or the pattern of actin filaments unambiguously identify all DCLK1-expressing cells as tuft cells. Figure 2. DCLK1-expressing tuft cells are post-mitotic and continuously renewed. (A) Immunofluorescent staining for COX1, DCLK1, PCNA, and Hoechst. The PCNA− nucleus of a tuft cell is highlighted by a yellow dotted circle. (B) Experimental scheme of the BrdU birth dating experiment. Relative proportion and number of crypt DCLK1-expressing cells positive for BrdU are indicated. Two representative immunofluorescent stainings for DCLK1 and BrdU are shown for the indicated time point. Nuclei are stained with Hoechst (blue). Yellow dotted circles highlight tuft cell nuclei. DCLK1+ tuft cells are postmitotic and undergo permanent turnover fueled by Lgr5-expressing CBC stem cells If all DCLK1-expressing cells are indeed differentiated cells, we would expect them to have a turnover rate similar to that of the other cell types of the intestinal epithelium, and, like other intestinal epithelial cell types, they should originate from Lgr5+ CBC stem cells. To measure their turnover rate, we birth-dated the DCLK1+ tuft cell population with BrdU. Wild-type C57BL/6 mice were treated with BrdU in drinking water for 1 or 2 wk. After 1 wk of BrdU treatment, we found that 96% DCLK1+ crypt tuft cells were BrdU+. This proportion reached 100% after 2 wk of BrdU treatment (Fig. 2 B). After 2 wk of BrdU treatment, 93% and 100% of tuft cells were BrdU− after 1 and 2 wk of chase with normal drinking water, respectively (Fig. 2 B). Therefore, DCLK1+ tuft cells are not quiescent stem cells but instead are postmitotic, short-lived differentiated cells, and their turnover time is close to 7 d. We then performed a lineage tracing experiment using the Lgr5-EGFP-IRES-CreERT2;Rosa26-LacZ compound knock-in mouse line, in which the expression of the LacZ reporter gene is prevented by a stop cassette flanked by LoxP sequences (Barker et al., 2007). Upon tamoxifen injection, CRE activity is induced in Lgr5+ stem cells and the stop cassette is excised, leading to permanent LacZ expression in the progeny of recombined Lgr5-expressing CBC stem cells. Of note, the intrinsically mosaic Cre expression in intestinal crypts of Lgr5-EGFP-IRES-CreERT2;Rosa26-LacZ mice results in a mosaic pattern of β-galactosidase in the corresponding villi (Fig. 3 A). If tuft cells originate from Lgr5-expressing stem cells, we would expect that the proportion of total β-galacosidase+ epithelial cells is identical to that of β-galactosidase+ tuft cells. When we quantified this, we found 53 β-galacosidase+ tuft cells out of 10,650 β-galactosidase+ epithelial cells (0.49%), and 51 β-galacosidase− tuft cells out of 14,340 β-galacosidase− epithelial cells (0.35%). The difference between the two values was not significant (P = 0.23), and both values were close to the representation of tuft cells in wild-type intestinal tissue sections (0.4%; see Fig. S2 A). In addition, tuft cells located within a stretch of β-galacosidase+ epithelial cells were invariably β-galacosidase+, as shown with SOX9 (Fig. 3 B) and DCLK1 (Fig. 3 C) stainings. This demonstrates that, like the four established differentiated cytotypes of the intestinal epithelium, tuft cells derive from Lgr5-expressing intestinal crypt base columnar stem cells. Figure 3. Tuft cells derive from Lgr5+ CBC stem cells. (A) Scheme explaining how chimeric Cre expression in crypts results in heterogeneous β-galactosidase staining in the adjacent villi (several crypts contribute to the generation of the cells constituting each villus). Wild-type (gray) and β-galactosidase (blue) cells coming from un-recombined (gray) and recombined (blue) crypts can migrate and colonize the same villus. The resulting cross section is shown. (B) Immunofluorescent staining for SOX9, β-galactosidase, β-catenin, and Hoechst in the Lgr5-EGFP-IRES-creERT2; Rosa26-LacZ mouse. Arrowheads point at SOX9+ tuft cells. The inset shows higher magnification of a SOX9+ tuft cells nucleus within a stretch of β-galactosidase+ cells. (C) Immunofluorescent staining for DCLK1, β-galactosidase, β-catenin, and Hoechst in intestinal sections from the Lgr5-EGFP-IRES-creERT2; Rosa26-LacZ mouse line. Arrowheads point at DCLK1+ tuft cells. The inset shows higher magnification of two DCLK1+ tuft cells within β-galactosidase+ crypts. β-galactosidase- crypts are shown by white dotted lines. Bars, 10 µm. Differentiated tuft cells appear postnatally The first tuft cells appear around d 7 after birth and become readily detectable a week later (Fig. 4). In adult mice, tuft cells are scattered throughout the crypt and villus epithelium, and represent ∼0.4% of all epithelial cells (Fig. S2 A). In adult animals, representation of the tuft cell population is similar throughout the entire length of the small and large intestines (Fig. S2 B). Except for SOX9, also expressed in Paneth cells, tuft cells do not share expression of other markers with enterocytes, goblet, or enteroendocrine cells (Fig. S3), suggesting that they constitute a distinct differentiated cell type. Figure 4. Tuft cells appear after birth. Immunofluorescent staining for DCLK1 and PCNA in the developing small intestine of E18.5, P7, and P12 mice. Arrowheads point at DCLK1-expressing tuft cells. Nuclei are stained with Hoechst (blue). Bars, 10 µm. Presence of cells expressing tuft cell differentiation markers in intestinal tumors Because tuft cells are the only epithelial cells expressing the COX1 and COX2 enzymes in the healthy intestinal epithelium, and these enzymes are strongly linked to intestinal tumorigenesis (Wang and Dubois, 2010), we analyzed the status of tuft cells in mouse small intestinal and colon tumors originating from two different oncogenic initiating events. Surprisingly, tuft cells could still be identified in the transformed lesions of mice carrying a k-RasV12G activating mutation (Janssen et al., 2002; Fig. 5, A and B) or a mutation in the Adenomatosis polyposis coli (Apc) tumor suppressor gene (Colnot et al., 2004; Fig. 5, C–F). In both cases, tuft cells invariably stained negative for expression of proliferation markers such as PCNA and did not incorporate BrdU (Fig. 5 F). In human lesions, tuft cells were also found in adenomas (Fig. 5 G), but rarely in adenocarcinoma (Fig. 5 H). This indicates that the tuft cell differentiation pathway is remarkably conserved in early tumor tissue, but not in their more malignant counterpart, raising the question of the potential role played by tuft cells during tumorigenesis. Figure 5. Tuft cells are found in mouse and human intestinal tumors. Immunofluorescent staining for tuft cells in K-RasV12G mouse adenoma (A and B); ApcΔ14 mouse adenoma (C–F), human adenoma (G), and human adenocarcinoma (H). Large fields (A and C) show clusters of tuft cells within the lesions. The lesion is delimited by PCNA (A) or β-catenin staining (C). DCLK1+ tuft cells coexpress the COX1 enzyme (B and D), show nuclear translocation of β-catenin (E), and are not in a proliferative state (F). Using HPGDS staining, tuft cells can also be detected in human adenomas (G) and, in rare cases, in restricted areas of human adenocarcinomas (H). For fluorescent staining, overlay (left) and individual signals of the indicated markers (right) are shown. Yellow dotted circles in E highlight tuft cell nuclei. Arrowheads point at tuft cells identified by DCLK1, COX1, and HPGDS expression. Nuclei are stained with Hoechst (blue) or hematoxylin (G and H). Bars: (A–F) 10 µm; (G and H) 100 µm. The ATOH1 transcription factor is required for tuft cell differentiation, which identifies them as secretory cells Having shown that tuft cells are differentiated and constantly renewed, we then asked to what extent they share the differentiation pathways previously reported for enterocytes, goblet, Paneth, and enteroendocrine cells and, therefore, to what extent tuft cells are related to these four cell types. It is generally assumed that commitment toward a specific differentiated cytotype involves the choice between one of the secretory fates (goblet, Paneth, or enteroendocrine cells) and the absorptive fate (enterocyte). This early cell fate decision is controlled by ATOH1 because differentiation of all secretory cell types is impaired in Atoh1 knock-out mice, whereas differentiation of the absorptive enterocytes is unaffected (Shroyer et al., 2007). Compared with Atoh1LoxP/LoxP or Villin-CreERT2 control mice (n = 5), in which tuft cell representation is unchanged, the intestinal epithelium of tamoxifen-injected Atoh1LoxP/LoxP; Villin-CreERT2 mice (n = 5) is completely devoid of tuft cells (Fig. 6). This conclusion was based on the analysis of several markers of tuft cells, including the SOX9 transcription factor and the COX1 enzyme (Fig. 6, A and C) and the α-tubulin and DCLK1 proteins (Fig. 6, B and D), which are related to the unique morphology of tuft cells. Thus, together with goblet, Paneth, and enteroendocrine cells, tuft cells depend on Atoh1 function for their differentiation and belong to a secretory lineage of the intestinal epithelium. Figure 6. Atoh1 is required for tuft cell differentiation. Immunofluorescent staining for the SOX9 transcription factor (A and C), the COX1 enzyme (A–D), and for the structural- and morphological-related tuft cells markers DCLK1 and α-tubulin (B and D) in intestines from control (A and B) and Atoh1-deficient mice (C and D), 3 wk after tamoxifen injection. Each panel contains the merged image on the left, and separate pictures of the indicated markers corresponding to the yellow inset on the right. Yellow arrowheads point at tuft cells revealed by SOX9 and COX1 or DCLK1, α-tubulin, and COX1 expression. Nuclei are stained with Hoechst (blue). Bars, 10 µm. Distinct genetic requirements for differentiation between tuft and enteroendocrine cells Tuft cells have recently been proposed to represent a subset of enteroendocrine cells (Formeister et al., 2009; Kokrashvili et al., 2009). To test this possibility, we checked whether Neurog3, a transcription factor essential for all enteroendocrine subtypes (Jenny et al., 2002; Mellitzer et al., 2010), is also required for tuft cells. We analyzed the tuft cell population in the intestinal epithelium of Neurog3LoxP/LoxP; Villin-Cre mice (n = 4), in which the Neurog3 gene is constitutively deleted at mid-gestation in all intestinal epithelial cells, and in control Neurog3LoxP/LoxP mice (n = 4). DCLK1 and chromogranin A (ChgA) immunohistochemical staining of sections from Neurog3-deficient mice and littermate controls showed that tuft cells are still present in the absence of Neurog3, whereas no enteroendocrine cells are found (Fig. 7, A and B). This indicates that tuft and enteroendocrine cells do not share the same transcription factor requirements for differentiation. Figure 7. Neurog3 is dispensable for tuft cell differentiation. (A and B) Immunofluorescent staining for DCLK1 and ChgA expression in intestines from 6-mo-old control or Neurog3-deficient mice. Yellow and green arrowheads point at tuft and enteroendocrine cells, respectively. (C) Immunofluorescent staining for SOX9 and Neurog3 in wild-type mouse intestine. The arrowhead points at a Neurog3+, SOX9− cell. Nuclei are stained with Hoechst (blue). Bars, 10 µm. Furthermore, within the crypt progenitor cell compartment, Neurog3 expression was restricted to enteroendocrine progenitor cells, in which SOX9 is barely detectable, in contrast to tuft cells where SOX9 is highly expressed (Fig. 7 C). These data, and the lack of evidence for an endocrine function of tuft cells, lead to the conclusion that tuft cells do not represent a subset of enteroendocrine cells. Distinct genetic requirements for differentiation between tuft and Paneth or goblet cells Goblet and Paneth cells share some transcription factor requirements for differentiation, for instance GFI1 (Shroyer et al., 2005; Bjerknes and Cheng, 2010) and SPDEF (Gregorieff et al., 2009; Noah et al., 2010). To investigate the relationship between these two cell types and tuft cells, we first analyzed tuft cell differentiation in Gfi1-deficient mice (Shroyer et al., 2005). In such mice (n = 5), we observed the expected increased representation of Neurog3+ and/or ChgA+ cells (Fig. S4 A), at the expense of goblet and Paneth cells (Shroyer et al., 2005). As previously reported (Bjerknes and Cheng, 2010), we also observed de novo Neurog3 expression and the presence of ChgA+ granules in some Gfi1-deficient lysozyme+ Paneth cells (Fig. S4 B). In contrast, the tuft cell population was not affected and Neurog3 expression was never detected in tuft cells (n = 126) from Gfi1-deficient mice (Fig. S4, A and B). This indicates that, unlike Paneth and goblet cells, tuft cells do not require GFI1 to repress Neurog3 expression. Second, we examined whether tuft cell differentiation requires SPDEF function. Similar numbers of tuft cells were identified after staining sections of intestine from Spdef-deficient and control mice (Fig. S4 C). This indicates that tuft cells can still differentiate in the absence of Spdef, which again distinguishes them from goblet and Paneth cells. Finally, it was shown previously that the SOX9 transcription factor is expressed in terminally differentiated Paneth cells (Blache et al., 2004) and is required for their differentiation (Bastide et al., 2007; Mori-Akiyama et al., 2007). The finding that post-mitotic tuft cells also express SOX9 prompted us to test whether it is required for their differentiation. Tamoxifen was injected to induce deletion of Sox9 and the mice were analyzed after various times, ranging from 1 wk to 4 wk after induction, the latest time corresponding to at least four complete renewal cycles of the intestinal epithelium. Independently of the time left between the tamoxifen injection and the analysis, tuft cells were still present in Sox9LoxP/LoxP; Villin-CreERT2 mice (n = 2), as evidenced by COX1 staining (Fig. 8 A), and still displayed their typical villin-immunoreactive apical tuft (Fig. 8 B) and DCLK1 expression (not depicted). Thus, SOX9 is not necessary for tuft cell survival and differentiation, nor for the expression of the COX1 and DCLK1 proteins. Figure 8. Normal tuft cell differentiation in Sox9-deficient intestine. (A) Immunofluorescent staining for SOX9 and COX1 in intestines from control or Sox9-deficient mice, 1 mo after the first tamoxifen injection. Yellow arrowheads point at tuft cells identified by SOX9 and/or COX1 expression. The right inset shows the gray level picture of the COX1 staining, which is hardly visible in the merged image. (B) Immunofluorescent staining for SOX9, villin, and COX1 in the intestines of wild-type and Sox9-deficient mice. Each panel contains the merged image on the left, and individual fluorescent signals of the indicated markers corresponding to the yellow inset on the right. Arrowheads point at tuft cells identified by SOX9 and/or COX1 and villin expression. The tuft cell nucleus shown in the Sox9-deficient tissue is highlighted by the yellow circle. Nuclei are stained with Hoechst (blue). Bars, 10 µm. Taken together, these data show that most of the well-known genetic factors that control differentiation of goblet and Paneth cells are not essential for differentiation of tuft cells, which suggests that tuft cells are not closely related to either of these two cell types. Tuft cells are responsible for opioid production by the intestinal epithelium Endogenous opioids mediate multiple functions in the regulation of the gastrointestinal mucosa physiology, including regulation of gastric emptying, gut motility, intestinal secretion, and pain (Holzer, 2009), and their production was recently reported to rely on a subpopulation of enteroendocrine cells (Kokrashvili et al., 2009) expressing the TRPM5 ion channel, but not ChgA, a marker of most enteroendocrine cells. As tuft cells are sometimes considered as enteroendocrine cells, and Trpm5 expression has been reported in tuft cells (Bezençon et al., 2008), Kokrashvili et al. (2009) hypothesized that opioid-producing cells could be tuft cells. We tested this hypothesis using our tuft cell markers. Indeed, costaining of intestinal villi for β-endorphin, COX1, and villin expression confirmed that β-endorphin production is restricted to tuft cells (Fig. 9). Importantly, all tuft cells express β-endorphin (n = 70) in the intestinal epithelium, thus validating the first tuft cell–specific functional property identified so far. Figure 9. Tuft cells are responsible for opioid production by the intestinal epithelium. Whole-mount immunofluorescent staining for β-endorphin, COX1, and villin in dissociated fragments of villus epithelium. Arrowheads point at tuft cells. Nuclei are stained with Hoechst (blue). Bars, 10 µm. Discussion Intestinal tuft cells have long been refractory to functional analyses, and until now could only be formally identified by electron microscopic analysis. In this paper we report several molecular markers that allow unambiguous identification of tuft cells of the intestinal epithelium, as well as in other organs (see Fig. S1), thus paving the way to their characterization. Development of tuft cells Tuft cells can be identified by DCLK1 expression from 1 wk postnatal in the mouse intestine. This is consistent with a study in the rat stomach, in which tuft cells are detected from the weaning stage (4 wk postnatal; Iseki et al., 1991). However, it cannot be excluded that immature tuft cells that do not express differentiation markers such as DCLK1 exist at earlier stages. For instance, tuft cells have been identified in the 20-wk-old fetal human small intestine, but their DCLK1 expression status has not been analyzed (Moxey and Trier, 1978). Early differentiation of tuft cells in crypt bottoms In the mouse small intestine, most tuft cells are found on villi or near the crypt–villus junction, but some are present in intestinal crypts where they can be detected at virtually any position above the Paneth cell compartment. Even when localized in crypts, identifiable tuft cells are postmitotic, always express differentiation markers such as DCLK1, HPGDS, and strong SOX9 expression, have elevated F-actin immunoreactivity, and display a gradient expression of COX1 and COX2 (exemplified for COX1 in Fig. 8 A). The presence of DCLK1+, COX1low in crypt bottoms suggests that, like for the enteroendocrine cell lineage (Fig. S5 and Bjerknes and Cheng, 2006), tuft cell fate commitment may occur at an early stage, in the close vicinity of or even within the stem cell compartment. DCLK1, quiescent stem cells, and tuft cells The fact that crypt base–located post-mitotic tuft cells express none of the common markers of the other, best known, lineages probably explains why DCLK1-expressing cells have previously been proposed as quiescent stem cells (Giannakis et al., 2006; May et al., 2008, 2009; Dekaney et al., 2009; Jin et al., 2009; Sureban et al., 2009). However, the majority of DCLK1+ cells are found in the villi, not in the stem cell compartment. In this study we confirmed DCLK1 as a specific marker of tuft cells. This permanently renewed cell population with a high turnover rate can hardly be considered as a likely source of quiescent stem cells, at least in healthy conditions. However, self-renewing stem cells and early differentiated precursor cell populations may not be sharply and definitely distinguished during the dynamics of epithelial turnover. For instance, tracing the progeny of Neurog3-expressing cells with a LacZ reporter gene could, in some rare events, identify complete β-galactosidase+ crypt–villus axes, indicating that Neurog3-controlled Cre expression occurred in pluripotent cells of the crypt, or that Neurog3-expressing enteroendocrine precursor cells reverted to a pluripotent state (Schonhoff et al., 2004). In our case, DCLK1 expression was only detected in post-mitotic cells, but it is not possible to definitively exclude that some early tuft cells that are still in the vicinity of the stem cell niche might have the capacity to de-differentiate and revert to a stem cell phenotype under stress conditions, which could potentially explain the results found by others using in vitro culture assays (May et al., 2009). Tuft cells represent a fourth secretory cell type of the intestinal epithelium Although tuft cells were identified 50 years ago, they have remained poorly characterized. As a result, the relationship between tuft cells and the four other cell types of the intestinal epithelium has remained elusive, and it is currently assumed that only four main differentiated cell types constitute this epithelium. Studies on the lineage of intestinal epithelial cells are still scarce, but pioneering experiments established that multipotent Lgr5-expressing CBC stem cells (Barker et al., 2007) produce several types of intermediate bipotent or monopotent precursors from which enterocyte or goblet cells (Bjerknes and Cheng, 1999) and enteroendocrine cells (Bjerknes and Cheng, 2006) arise. In addition, multiple gene deletion studies in mouse models facilitated the decoding of transcription factor requirements for differentiation of these four cell types, but tuft cells have never been considered in such studies. Here, we evaluated the status of tuft cells in several of these genetically engineered mouse lines. We found that tuft cells are absent in Atoh1-deficient mice, which according to the prevalent model of intestinal epithelial differentiation (van der Flier and Clevers, 2009), characterizes them as a secretory cell type. This is consistent with their capacity to produce (Fig. 9) and release opioids through an exocrine–paracrine mechanism (Kokrashvili et al., 2009). However, the fact that previous reports included tuft cells in the enteroendocrine lineage (Formeister et al., 2009; Kokrashvili et al., 2009) prompted us to investigate whether one or several differentiation pathways are shared between enteroendocrine and tuft cells. We found that in contrast to the enteroendocrine cell lineage, which strictly depends on Neurog3 function (Jenny et al., 2002; Mellitzer et al., 2010), tuft cells are still produced in the absence of Neurog3. In addition, COX1low tuft cells that are still in the process of terminal maturation express high levels of SOX9 and never express Neurog3, whereas SOX9 is barely detectable in Neurog3-expressing enteroendocrine precursor cells (Bjerknes and Cheng, 2006). Tuft cells and Ngn3-dependent enteroendocrine cells thus constitute distinct cell types. In the future, however, it is conceivable that an endocrine type of secretion is demonstrated for tuft cells. In this case, they could be considered as type 2 enteroendocrine cells, which do not require Ngn3 function for their differentiation. Moreover, whereas differentiation of both goblet and Paneth cells is regulated by GFI1 (Shroyer et al., 2005; Bjerknes and Cheng, 2010) and SPDEF (Gregorieff et al., 2009; Noah et al., 2010), these transcription factors are not essential for tuft cells. In addition, Paneth cells require SOX9 function to differentiate (Bastide et al., 2007; Mori-Akiyama et al., 2007) but tuft cells, which express high levels of SOX9 in their terminally differentiated state, are still present in Sox9-deficient intestinal epithelium. In summary, although tuft cells are produced from the same Lgr5+ stem cell as enterocytes, goblet, Paneth, and enteroendocrine cells, we show here that they represent an independent cell type: (i) they express a specific marker signature, and (ii) except for ATOH1, none of the transcription factors known to be required for differentiation of the other intestinal epithelial cell types and that we tested here is essential for tuft cell differentiation (Fig. 10). Figure 10. Updated model for the differentiation of the intestinal epithelial cell types. The scheme on the left represents a crypt–villus unit in the adult mouse small intestinal epithelium. The main functions, including the recently discovered function of Paneth cells in maintaining the CBC stem cell population (Sato et al., 2011), and representative molecular markers identifying each of the cell types and the intestinal stem cell are indicated. Opioid secretion is known to occur in the gut lumen (blue arrows; see Kokrashvili et al., 2009). Strong evidence suggests that tuft cells can also act as an epithelial source of prostanoids (Bezençon et al., 2008 and this paper), but the underlying secretion mechanism still has to be demonstrated. The diagram on the right summarizes the genetic hierarchy of epithelial cell lineage commitment in the intestine. Intestinal CBC stem cells proliferate and produce progenitors. Choice between absorptive or secretory cell fates is under the control of the hairy/enhancer of split 1 (Hes1) or atonal homologue 1 (Atoh1) gene. Within the cells committed to secretory types, Neurog3 is required for enteroendocrine cell differentiation. Gfi1 is required for Paneth and goblet cell differentiation, preventing the expression of Neurog3. Sox9 is essential for differentiation of Paneth cells. Spdef is required for both Paneth and goblet cell terminal maturation. M-cells are known to derive from Lgr5+ CBC stem cells (Barker and Clevers, 2010), but knowledge of the molecular pathways leading to their differentiation is still missing. Future studies will deepen the knowledge of the lineage intermediates that are shared by precursors of several differentiated cell types and refine the current understanding of the relationship between tuft cells and the other constituents of the intestinal epithelium. Understanding the role played by tuft cells in the physiopathology of the intestinal epithelium is currently hampered by the absence of animal models specifically devoid of tuft cells. As previously suggested by gene profiling experiments in intestinal Trpm5-expressing cells (Bezençon et al., 2008), we confirm here that tuft cells are the only epithelial cells in the healthy mucosa to express the COX1 and COX2 enzymes, whose expression is rate limiting for the biosynthesis of prostanoids. Tuft cells also express Hpgds and thus represent a likely epithelial source of prostaglandin-D2. This is important given the central role played by prostanoids in mediating inflammation and tumorigenesis in the intestinal epithelium (Stenson, 2008; Wang and Dubois, 2010). Indeed, the presence of tuft cell clusters in tumors from Apc- or K-Ras–mutated mice suggests a possible contribution of tuft cells during tumorigenesis. The previous observation that siRNA-mediated inhibition of Dclk1 expression in tumor xenografts resulted in reduced tumor growth (Sureban et al., 2009) further supports this notion. Finally, we show here that tuft cells are the only intestinal epithelial cells to produce β-endorphin in healthy conditions, and thus likely contribute to the regulation of vasoconstriction, peristaltic movements, and pain in the intestine. In conclusion, we present here molecular and genetic evidences that tuft cells constitute a genuine fifth cell type in the intestinal epithelium, as well as the first insights into functions of these cells with their production of prostanoids and opioids. Further studies will determine whether tuft cells from other endoderm-derived epithelia share similar functions. Materials and methods Animals: mouse strains The Sox9LoxP/LoxP; Villin-CreERT2 strain was obtained by mating Sox9LoxP/LoxP mice (Kist et al., 2002) with Villin-CreERT2 animals (el Marjou et al., 2004). Sox9 deletion in the intestinal epithelium was induced by a single daily i.p. injection of 1 mg tamoxifen (Sigma-Aldrich) for 5 d. Mice were sacrificed 1 wk and 1 mo after the first tamoxifen injection. The ApcΔ14 (Colnot et al., 2004), Villin-K-RasV12G (Janssen et al., 2002), Neurog3LoxP/LoxP; Villin-Cre (Mellitzer et al., 2010), Gfi1 −/− (Shroyer et al., 2005), and Spdef −/− (Gregorieff et al., 2009) strains have been described previously. The Lgr5-EGFP-IRES-creERT2; Rosa26-LacZ strain has been described previously (Barker et al., 2007); mice were induced at 4 wk of age by a single i.p. injection of tamoxifen, and were analyzed 14 or 22 mo later. Atoh1LoxP/LoxP; Villin-CreERT2 mice were obtained by crossing Atoh1LoxP/LoxP (Shroyer et al., 2007) and Villin-CreERT2 animals (el Marjou et al., 2004). Atoh1 deletion was induced by a single daily i.p. injection of 1 mg tamoxifen (Sigma-Aldrich) for 4 d. Mice were sacrificed on d 5 or 22 after the first tamoxifen injection. Wild-type animals used in this study had a C57BL/6 genetic background. For proliferation analyses, mice were injected with 0.1 mg BrdU (Roche)/PBS per gram of mouse body weight. Mice were sacrificed 2 h after injection. For long-term BrdU incorporation studies, BrdU was given ad libitum (0.5 mg/ml) during the indicated time. Human samples Slides of human intestinal biopsies were kindly provided by Drs. J.-F. Bourgaux and C. Pignodel (Centre Hospitalier Régional Universitaire, Nîmes, France). Fluorescent and bright-field immunohistochemistry on paraffin-embedded tissue Tissue dissection, fixation, and immunohistochemistry on thin sections of paraffin-embedded tissue were performed essentially as described previously (Bastide et al., 2007). In brief, 5-µm-thick sections were dewaxed in xylene and rehydrated in graded alcohol baths. Antigen retrieval was performed by boiling slides for 20 min in 10 mM sodium citrate buffer, pH 6.0. Nonspecific binding sites were blocked in blocking buffer (TBS, pH 7.4, 5% dried milk, and 0.5% Triton X-100) for 60 min at RT. Sections were then incubated with primary antibodies diluted in blocking buffer overnight at 4°C. Primary antibodies used in this study were as follows: anti-SOX9 (AB5535; 1:1,000) and anti-villin (MAB1671; 1:500) were purchased from Millipore. Anti-COX1 (sc-1754; 1:200), anti-COX2 (sc-1747; 1:200), anti-PCNA (sc-56; 1:200), and anti-MUC2 (sc-15334; 1:200) were from Santa Cruz Biotechnology, Inc. Anti-HPGDS (160013; 1:200) was from Cayman Chemical. Anti–α-tubulin (32–2500; 1:200) was from Invitrogen. Anti-Neurog3 (F25A1B3; 1:100) was from the Developmental Studies Hybridoma Bank (Iowa City, IA). Anti–β-catenin (610154; 1:200) was from BD. Anti–β-endorphin (20063; 1:100) was from Immunostar. Anti-BrdU was from Abcam (Ab1893; 1:300) or from the Developmental Studies Hybridoma Bank (G3G4; 1:300). Anti–β-galactosidase (ab9361; 1:400) was from Abcam. Anti-lysozyme (RB-372; 1:500) was from NeoMarkers. Anti-DCLK1 was from Abcam (ab37994; 1:200) or Abgent (AP7219b; 1:200). Anti-ChgA was from Immunostar (20085; 1:1,000) or Santa Cruz Biotechnology, Inc. (sc-1488; 1:400). Anti-Ki67 (ab15580; 1:500) and anti–phospho-histone H3 (ab14955; 1:800) were from Abcam. Slides were then washed two times with 0.1% PBS-Tween (Sigma-Aldrich) before incubation with fluorescent secondary antibodies conjugated with either Alexa 488, Cyanin-3, or Cyanin-5 (Jackson ImmunoResearch Laboratories, Inc.) and Hoechst at 2 µg/ml (Sigma-Aldrich) in PBS–Triton X-100 0.1% (Sigma-Aldrich). Stained slides were then washed two extra times in PBS before mounting with aqueous glycerol–mowiol medium. Methods used for bright-field immunohistochemistry were identical, except that slides were incubated with 1.5% H2O2 in methanol for 20 min and washed in PBS to quench endogenous peroxydase activity before antigen retrieval. Envision+ (Dako) was used as a secondary reagent. Signals were developed with DAB (Sigma-Aldrich) and a hematoxylin counterstain (DiaPath) was used. After dehydration, sections were mounted in Pertex (Histolab). Whole-mount fluorescent immunohistochemistry For some experiments, immunolabeling was performed directly on whole crypts or whole pieces of villus epithelium. In brief, the small intestine was removed, flushed with PBS, and turned inside-out on a wooden stick before being incubated in 10 mM DTT for 15 min at room temperature to remove mucus. Tissue was then incubated in cold Ca2+/Mg2+-free PBS containing 10 mM EDTA for 20 min and nearly intact pieces of villus epithelium were recovered by gentle shaking. Cycles of incubation/shaking were repeated until whole crypts were obtained. Whole fragments of epithelium were fixed in 4% PFA for 2 h at RT and then washed twice in PBS before being processed for immunohistochemistry. Pieces of epithelium were first incubated and permeabilized in blocking buffer (TBS, pH 7.4; 5% dried milk, and 0.5% Triton X-100) for 1 h at RT before incubation with primary antibodies overnight. Material was then washed four times in PBS Triton X-100 (0.1%) at RT before incubation with fluorescent-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.) and Hoechst at 2 µg/ml (Sigma-Aldrich) for 60 min at RT in PBS Triton X-100 (0.1%). Stained tissues were washed two extra times in PBS, smeared in poly-lysine–coated slides, and mounted in glycerol–mowiol medium. F-actin staining was performed with Alexa Fluor 647–conjugated phalloidin (Invitrogen). Microscopy and imaging Fluorescent pictures were acquired at room temperature under a microscope (AxioImager Z1; Carl Zeiss, Inc.), equipped with a camera (AxioCam MRm; Carl Zeiss, Inc.), EC Plan Neofluar (5x, NA 0.16; 10x, NA 0.3; 20x, 0.5 NA; 100x, NA 1.3) and Plan Apochromat (40x, NA 0.95; 63x, NA 1.4) objectives, the Apotome Slider system equipped with an H1 transmission grid (Carl Zeiss, Inc.), and AxioVision software (Carl Zeiss, Inc.). Aqueous mounting medium (0.1 M Tris, pH 8.5, 24% glycerol wt/vol, 10% mowiol wt/vol [Sigma-Aldrich], and 2.5 mg.ml−1 final DABCO [Sigma-Aldrich]) was used for fluorescent acquisition. Bright-field immunohistochemistry pictures were taken at room temperature with a microscope (Eclipse 80i; Nikon) with Plan Fluor (10x, NA 0.3; 20x, NA 0.5; 40x, NA 0.75; and 60x, NA 0.5–1.25) lenses (Nikon) and a digital camera (Q-Imaging Retiga 2000R with a Q-Imaging RGB Slider). Pictures were captured with Q-Capture Pro software (Nikon). For wide-field acquisition of bright-field images, slides were scanned at 20x with the Nanozoomer Slide Scanner (Hamamatsu Photonics). Resulting images were visualized and annotated with NDPview software (Hamamatsu Photonics). Post-treatment of pictures, annotations, and panel composition were performed with Photoshop software (Adobe). Statistical analyses Histograms and determination of standard deviations were calculated with Excel software (Microsoft). Statistical analysis of proportions of β-galactosidase+ tuft cells versus β-galactosidase+ epithelial cells was performed by a Mann-Whitney test with Prism software (GraphPad Software, Inc.). Online supplemental material Fig. S1 shows identification of tuft cells in mouse and human tissues, using the tuft cell signature. Fig. S2 shows that tuft cells are found all along the intestinal tract. Fig. S3 shows that intestinal tuft cells do not share terminal differentiation markers with the other secretory cell lineages. Fig. S4 shows that the tuft cell population is not affected in Gfi-1– or Spdef-deficient mice. Fig. S5 shows early enteroendocrine cell differentiation in intestinal crypts. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201010127/DC1.
Author and article information
Journal
Journal ID (nlm-ta): J Cell Biol
Journal ID (hwp): jcb
Title:
The Journal of Cell Biology
Publisher:
The Rockefeller University Press
ISSN
(Print):
0021-9525
ISSN
(Electronic):
1540-8140
Publication date
(Print):
7
March
2011
Volume: 192
Issue: 5
Page: 706
Author notes
Article
Publisher ID: jcb.1925iti2DOI: 10.1083/jcb.1925iti2
PMC ID: 3051811
SO-VID: 020bd41b-3a28-4dd8-8cdb-886ab5751932
Copyright © © 2011 The Rockefeller University Press
History
Categories
Subject:
News
Subject:
In This Issue
ScienceOpen disciplines: Cell biology
Data availability:
ScienceOpen disciplines: Cell biology
