- Record: found
- Abstract: found
- Article: found
Anticoagulant therapy in patients with liver cirrhosis and portal vein thrombosis: insights for the clinician
Read this article at
Abstract
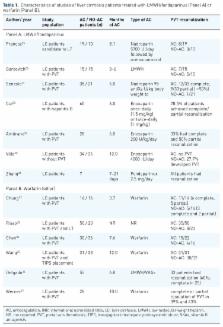
Portal vein thrombosis (PVT) is a frequent complication in the natural history of patients with liver cirrhosis (LC). The prevalence of PVT in LC is highly variable, ranging from 0.6% to 25% according to different reports. The impact of PVT on the natural history of LC is unclear, but it seems to negatively affect the prognosis of patients undergoing liver transplantation (LT) by increasing post-LT mortality and delaying waiting time. The antithrombotic treatment of PVT is still challenging as PVT may often remain asymptomatic and incidentally diagnosed, and a spontaneous partial/total regression of PVT is observed in an important proportion of patients, even in the absence of anticoagulation. Recent evidence suggested that the anticoagulant treatment for PVT may favorably affect both ischemic and bleeding outcomes in LC patients. Anticoagulant therapies so far available include unfractioned heparin, low molecular weight heparins (LMWHs) and fondaparinux for acute treatment, and LMWHs and vitamin K antagonists (VKAs) for long-term treatment. No robust data currently support the use of direct oral anticoagulants (DOACs) in patients with LC and PVT, as the safety and efficacy of DOACs in this setting is still unclear. This review summarizes current evidence for the evaluation and management of patients with LC and PVT.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis.
- Record: found
- Abstract: found
- Article: not found
Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study.
- Record: found
- Abstract: found
- Article: not found