- Record: found
- Abstract: found
- Article: found
Fear response-based prediction for stress susceptibility to PTSD-like phenotypes
Read this article at
Abstract
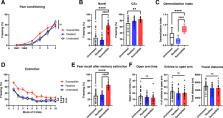
Most individuals undergo traumatic stresses at some points in their life, but only a small proportion develop stress-related disorders such as anxiety diseases and posttraumatic stress disorder (PTSD). Although stress susceptibility is one determinant of mental disorders, the underlying mechanisms and functional implication remain unclear yet. We found that an increased amount of freezing that animals exhibited in the intertrial interval (ITI) of a stress-enhanced fear learning paradigm, predicts ensuing PTSD-like symptoms whereas resilient mice show ITI freezing comparable to that of unstressed mice. To examine the behavioral features, we developed a systematic analytical approach for ITI freezing and stress susceptibility. Thus, we provide a behavioral parameter for prognosis to stress susceptibility of individuals in the development of PTSD-like symptoms as well as a new mathematical means to scrutinize freezing behavior.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
MicroRNA as repressors of stress-induced anxiety: the case of amygdalar miR-34.
- Record: found
- Abstract: found
- Article: not found
Single prolonged stress disrupts retention of extinguished fear in rats.
- Record: found
- Abstract: found
- Article: not found