- Record: found
- Abstract: found
- Article: found
A Cross-Sectional Study Assessing Appropriateness Of Inhaled Corticosteroid Treatment In Primary And Secondary Care Patients With COPD In Sweden
Abstract
Purpose
Inhaled corticosteroids (ICS) are often more widely prescribed in the treatment of chronic obstructive pulmonary disease (COPD) than what is recommended in the guidelines. The aim of this study was to evaluate the appropriateness of ICS treatment in COPD patients using the algorithm proposed by the International Primary Care Respiratory Group (IPCRG) and to identify factors associated with ICS treatment.
Patients and methods
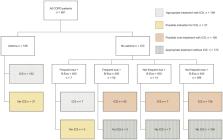
Appropriateness of ICS therapy was studied with respect to concomitant asthma, history of exacerbations and blood eosinophils (B-Eos) in a Swedish cohort of primary and secondary care patients with COPD. Factors associated with ICS were investigated using multivariable logistic regression.
Results
Triple treatment was found to be the most common treatment combination, used by 46% of the 561 included patients, and in total 63% were using ICS. When applying the IPCRG algorithm, there was a possible indication for discontinuation of ICS in 55% of the patients with ICS treatment. Of the patients not using ICS, 18% had an indication for starting such treatment. The strongest factors associated with ICS therapy were frequent exacerbations (aOR 8.61, 95% CI 4.06, 20.67), secondary care contacts (aOR 6.99, 95% CI 2.48, 25.28) and very severe airflow limitation (aOR 5.91, 95% CI 1.53, 26.58).
Conclusion
More than half of the COPD patients on ICS met the criteria where withdrawal of the treatment could be tried. There was, however, also a subgroup of patients not using ICS for whom there was an indication for starting ICS treatment. Patients using ICS were characterized by more frequent exacerbations and lower lung function.
Most cited references10
- Record: found
- Abstract: found
- Article: not found
Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials.
- Record: found
- Abstract: found
- Article: not found
Randomized controlled trial of pulmonary rehabilitation in severe chronic obstructive pulmonary disease patients, stratified with the MRC dyspnoea scale.
- Record: found
- Abstract: found
- Article: not found