- Record: found
- Abstract: found
- Article: found
Measurement of the Combined Levels of Serum Uric Acid and Alanine Aminotransferase and the Risk of Metabolic Syndrome in a Population Aged 60 Years or More in Northeastern China
Read this article at
Abstract
Background
Serum uric acid (SUA) and alanine aminotransferase (ALT) levels are increased in patients with metabolic syndrome. This study aimed to investigate the association between the combined levels of SUA and ALT and the risk of metabolic syndrome in residents ≥60 years of age in Northeastern China.
Material/Methods
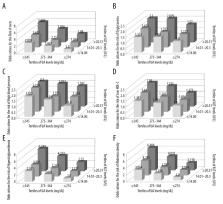
A population study included nine communities in Shenyang, Northeast China, and 3,998 participants (1,434 men and 2,564 women) who were ≥60 years old. SUA and ALT measurements (levels 1–3) and clinical parameters were recorded. Metabolic syndrome was diagnosed according to the criteria of the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III). The association between the combined SUA and ALT levels and metabolic syndrome was determined by multivariate logistic regression analysis in tertiles that included Groups 1–9.
Results
The prevalence of metabolic syndrome was 43.2% (men), and 61.9% (women), and the prevalence and odds ratio (OR) values increased with increasing SUA and ALT levels. The OR values of metabolic syndrome in the ALT Groups 2–3 were 1.329 (95% CI, 1.137–1.554) and 2.362 (95% CI, 2.006–2.781), and in the SUA Groups 2–3 the OR values were 1.718 (95% CI, 1.466–2.015) and 2.743 (95% CI, 2.310–3.256). The OR of the combined increase in SUA and ALT and metabolic syndrome in Groups 1–9 ranged from 1.494–5.889 (all, p<0.05).
Related collections
Most cited references36

- Record: found
- Abstract: found
- Article: found
Sugar, Uric Acid, and the Etiology of Diabetes and Obesity
- Record: found
- Abstract: found
- Article: not found
Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease.
- Record: found
- Abstract: found
- Article: not found