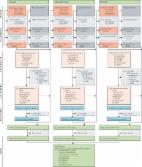
Summary Background Sharing of equipment used for injecting drug use (IDU) is a substantial cause of disease burden and a contributor to blood-borne virus transmission. We did a global multistage systematic review to identify the prevalence of IDU among people aged 15–64 years; sociodemographic characteristics of and risk factors for people who inject drugs (PWID); and the prevalence of HIV, hepatitis C virus (HCV), and hepatitis B virus (HBV) among PWID. Methods Consistent with the GATHER and PRISMA guidelines and without language restrictions, we systematically searched peer-reviewed databases (MEDLINE, Embase, and PsycINFO; articles published since 2008, latest searches in June, 2017), searched the grey literature (websites and databases, searches between April and August, 2016), and disseminated data requests to international experts and agencies (requests sent in October, 2016). We searched for data on IDU prevalence, characteristics of PWID, including gender, age, and sociodemographic and risk characteristics, and the prevalence of HIV, HCV, and HBV among PWID. Eligible data on prevalence of IDU, HIV antibody, HBsAg, and HCV antibody among PWID were selected and, where multiple estimates were available, pooled for each country via random effects meta-analysis. So too were eligible data on percentage of PWID who were female; younger than 25 years; recently homeless; ever arrested; ever incarcerated; who had recently engaged in sex work, sexual risk, or injecting risk; and whose main drugs injected were opioids or stimulants. We generated regional and global estimates in line with previous global reviews. Findings We reviewed 55 671 papers and reports, and extracted data from 1147 eligible records. Evidence of IDU was recorded in 179 of 206 countries or territories, which cover 99% of the population aged 15–64 years, an increase of 31 countries (mostly in sub-Saharan Africa and the Pacific Islands) since a review in 2008. IDU prevalence estimates were identified in 83 countries. We estimate that there are 15·6 million (95% uncertainty interval [UI] 10·2–23·7 million) PWID aged 15–64 years globally, with 3·2 million (1·6–5·1 million) women and 12·5 million (7·5–18·4 million) men. Gender composition varied by location: women were estimated to comprise 30·0% (95% UI 28·5–31·5) of PWID in North America and 33·4% (31·0–35·6) in Australasia, compared with 3·1% (2·1–4·1) in south Asia. Globally, we estimate that 17·8% (10·8–24·8) of PWID are living with HIV, 52·3% (42·4–62·1) are HCV-antibody positive, and 9·0% (5·1–13·2) are HBV surface antigen positive; there is substantial geographic variation in these levels. Globally, we estimate 82·9% (76·6–88·9) of PWID mainly inject opioids and 33·0% (24·3–42·0) mainly inject stimulants. We estimate that 27·9% (20·9–36·8) of PWID globally are younger than 25 years, 21·7% (15·8–27·9) had recently (within the past year) experienced homelessness or unstable housing, and 57·9% (50·5–65·2) had a history of incarceration. Interpretation We identified evidence of IDU in more countries than in 2008, with the new countries largely consisting of low-income and middle-income countries in Africa. Across all countries, a substantial number of PWID are living with HIV and HCV and are exposed to multiple adverse risk environments that increase health harms. Funding Australian National Drug and Alcohol Research Centre, Australian National Health and Medical Research Council, Open Society Foundation, World Health Organization, the Global Fund, and UNAIDS.