- Record: found
- Abstract: found
- Article: found
A Metabolic Signature of Mitochondrial Dysfunction Revealed through a Monogenic Form of Leigh Syndrome

Read this article at
SUMMARY
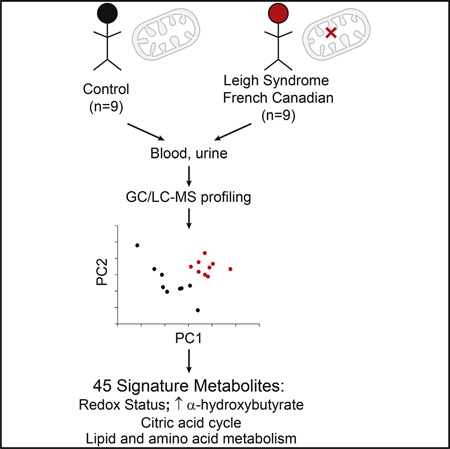
A decline in mitochondrial respiration represents the root cause of a large number of inborn errors of metabolism. It is also associated with common age-associated diseases and the aging process. To gain insight into the systemic, biochemical consequences of respiratory chain dysfunction, we performed a case-control, prospective metabolic profiling study in a genetically homogenous cohort of patients with Leigh syndrome French Canadian variant, a mitochondrial respiratory chain disease due to loss-of-function mutations in LRPPRC. We discovered 45 plasma and urinary analytes discriminating patients from controls, including classic markers of mitochondrial metabolic dysfunction (lactate and acylcarnitines), as well as unexpected markers of cardiometabolic risk (insulin and adiponectin), amino acid catabolism linked to NADH status (α-hydroxybutyrate), and NAD + biosynthesis (kynurenine and 3-hydroxyanthranilic acid). Our study identifies systemic, metabolic pathway derangements that can lie downstream of primary mitochondrial lesions, with implications for understanding how the organelle contributes to rare and common diseases.
Graphical abstract
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation
- Record: found
- Abstract: found
- Article: not found
Identification of a gene causing human cytochrome c oxidase deficiency by integrative genomics.

- Record: found
- Abstract: found
- Article: found