- Record: found
- Abstract: found
- Article: found
Muscle Contraction, but Not Insulin, Increases Microvascular Blood Volume in the Presence of Free Fatty Acid–Induced Insulin Resistance
Read this article at
Abstract
OBJECTIVE
Insulin and contraction each increase muscle microvascular blood volume (MBV) and glucose uptake. Inhibiting nitric oxide synthase blocks insulin's but not contraction's effects. We examined whether contraction could augment the MBV increase seen with physiologic hyperinsulinemia and whether free fatty acid (FFA)-induced insulin resistance differentially affects contraction- versus insulin-mediated increases in MBV.
RESEARCH DESIGN AND METHODS
Rats were fasted overnight. Plasma FFAs were increased by intralipid/heparin infusion (3 h), insulin was increased with a euglycemic clamp (3 mU · min −1 · kg −1), and hindlimb muscle contraction was electrically stimulated. Muscle MBV was measured using contrast-enhanced ultrasound. Insulin transport into muscle was measured using 125I-insulin. BQ-123 (0.4 mg/h) was used to block the endothelin-1 (ET-1) receptor A.
RESULTS
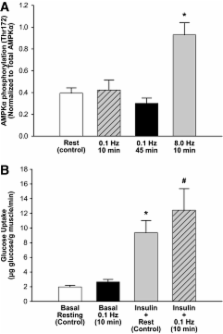
Superimposing contraction on physiologic hyperinsulinemia increased MBV within 10 min by 37 and 67% for 0.1 or 1 Hz, respectively ( P < 0.01). FFA elevation alone did not affect MBV, whereas 0.1 Hz stimulation doubled MBV ( P < 0.05) and increased muscle insulin uptake ( P < 0.05) despite high FFA. Physiologic hyperinsulinemia during FFA elevation paradoxically decreased MBV ( P < 0.05). This MBV decrease was reversed by either 0.1 Hz contraction or ET-1 receptor A antagonism, and the combination raised MBV above basal.
CONCLUSIONS
Contraction recruits microvasculature beyond that seen with physiologic hyperinsulinemia by a distinct mechanism that is not blocked by FFA-induced vascular insulin resistance. The paradoxical MBV decline seen with insulin plus FFA may result from differential inhibition of insulin-stimulated nitric oxide–dependent vasodilation relative to ET-1 vasoconstriction. Our results implicate ET-1 as a potential mediator of FFA-induced vascular insulin resistance.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo.
- Record: found
- Abstract: found
- Article: not found
Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus.
- Record: found
- Abstract: found
- Article: not found