- Record: found
- Abstract: found
- Article: found
The mechanism of hsa-miR-424-5 combining PD-1 through mTORC signaling pathway to stimulate immune effect and participate in Type 1 diabetes
Read this article at
Abstract
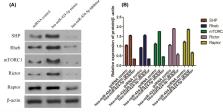
In the present study, hsa-miR-424-5p mimic plasmid and hsa-mir-424-5p inhibitor plasmid were designed and injected into rats respectively, and miRNA control plasmid was also constructed. Models of Type 1 diabetes (T1D) were built. After successful modeling, the expression of hsa-miR-424-5p in lymphocytes was analyzed by RT-PCR. The expression of protein PD-1, T-bet, CXCR3, STING in Th1 lymphocytes and content of IGF-1 in islet tissue were analyzed by flow analysis. The protein levels of SHP2, Rheb, mTORC1, Rictor and Raptor in islet tissue were analyzed by Western blot. The results showed that hsa-miR-424-5p mimic group had the highest expression of hsa-miR-424-5p in lymphocytes. The expression of PD-1 was in hsa-miR-424-5p inhibitor > miRNA control > hsa-miR-424-5p mimic, while the expression of T-bet, CXCR3 and STING was in hsa-miR-424-5p mimic > miRNA control > hsa-miR-424-5p inhibitor. The expression of IGF-1 protein in hsa-miR-424-5p inhibitor group was the highest (32.08%) and hardly expressed in hsa-miR-424-5p mimic group (2.36%). The expression of SHP2, Rheb, mTORC1, Rictor and Raptor of insulin histoproteins were in hsa-miR-424-5p mimic group > miRNA control of > hsa-miR-424-5p inhibitor group, with statistical differences. It indicates that hsa-miR-424-5p binding PD-1 signaling molecules can stimulate the immune effect through the mTORC signaling pathway and participates in the pathogenesis of T1D.
Related collections
Most cited references12
- Record: found
- Abstract: found
- Article: not found
mTORC2 Promotes Tumorigenesis via Lipid Synthesis
- Record: found
- Abstract: found
- Article: not found
Efficacy and Safety of Dapagliflozin in Patients With Inadequately Controlled Type 1 Diabetes (the DEPICT-2 Study): 24-Week Results From a Randomized Controlled Trial
- Record: found
- Abstract: found
- Article: not found