- Record: found
- Abstract: found
- Article: found
Non-Centered Spike-Triggered Covariance Analysis Reveals Neurotrophin-3 as a Developmental Regulator of Receptive Field Properties of ON-OFF Retinal Ganglion Cells
Read this article at
Abstract
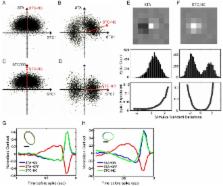
The functional separation of ON and OFF pathways, one of the fundamental features of the visual system, starts in the retina. During postnatal development, some retinal ganglion cells (RGCs) whose dendrites arborize in both ON and OFF sublaminae of the inner plexiform layer transform into RGCs with dendrites that monostratify in either the ON or OFF sublamina, acquiring final dendritic morphology in a subtype-dependent manner. Little is known about how the receptive field (RF) properties of ON, OFF, and ON-OFF RGCs mature during this time because of the lack of a reliable and efficient method to classify RGCs into these subtypes. To address this deficiency, we developed an innovative variant of Spike Triggered Covariance (STC) analysis, which we term Spike Triggered Covariance – Non-Centered (STC-NC) analysis. Using a multi-electrode array (MEA), we recorded the responses of a large population of mouse RGCs to a Gaussian white noise stimulus. As expected, the Spike-Triggered Average (STA) fails to identify responses driven by symmetric static nonlinearities such as those that underlie ON-OFF center RGC behavior. The STC-NC technique, in contrast, provides an efficient means to identify ON-OFF responses and quantify their RF center sizes accurately. Using this new tool, we find that RGCs gradually develop sensitivity to focal stimulation after eye opening, that the percentage of ON-OFF center cells decreases with age, and that RF centers of ON and ON-OFF cells become smaller. Importantly, we demonstrate for the first time that neurotrophin-3 (NT-3) regulates the development of physiological properties of ON-OFF center RGCs. Overexpression of NT-3 leads to the precocious maturation of RGC responsiveness and accelerates the developmental decrease of RF center size in ON-OFF cells. In summary, our study introduces STC-NC analysis which successfully identifies subtype RGCs and demonstrates how RF development relates to a neurotrophic driver in the retina.
Author Summary
The developmental separation of ON and OFF pathways is one of the fundamental features of the visual system. In the mouse retina, some bi-stratified ON-OFF RGCs are refined into mono-stratified ON or OFF RGCs during the first postnatal month. However, the process by which the RGCs' physiological receptive field properties mature remains incompletely characterized, mainly due to the lack of a reliable and efficient method to classify RGCs into different subtypes. Here we have developed an innovative analysis, Spike Triggered Covariance – Non-Centered (STC-NC), and demonstrated that this technique can accurately characterize the receptive field properties of ON, OFF and ON-OFF center cells. We show that, in wildtype mouse, RGCs gradually develop sensitivity to focal stimulation after eye opening, and the development of ON-OFF receptive field center properties correlates well with their dendritic laminar refinement. Furthermore, overexpression of NT-3 accelerates the developmental decrease of receptive field center size in ON-OFF cells. Our study is the first to establish the STC-NC analysis which can successfully identify ON-OFF subtype RGCs and to demonstrate how receptive field development relates to a neurotrophic driver in the retina.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
A simple white noise analysis of neuronal light responses.
- Record: found
- Abstract: found
- Article: not found
Do we know what the early visual system does?
- Record: found
- Abstract: found
- Article: not found