- Record: found
- Abstract: found
- Article: found
AMPKα1 confers survival advantage of colorectal cancer cells under metabolic stress by promoting redox balance through the regulation of glutathione reductase phosphorylation
Read this article at
Abstract
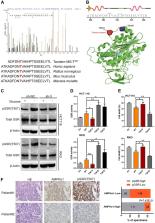
Patients with stage II or III colorectal cancer (CRC) exhibit various clinical outcomes after radical treatments. The 5-year survival rate was between 50 and 87%. However, the underlying mechanisms of the variation remain unclear. Here we show that AMPKα1 is overexpressed in CRC patient specimens and the high expression is correlated with poor patient survival. We further reveal a previously unrecognized function of AMPKα1, which maintains high level of reduced glutathione to keep reduction–oxidation reaction (redox) homeostasis under stress conditions, thus promoting CRC cell survival under metabolic stress in vitro and enhancing tumorigenesis in vivo. Mechanistically, AMPKα1 regulate the glutathione reductase (GSR) phosphorylation possibly through residue Thr507 which enhances its activity. Suppression of AMPKα1 by using nano-sized polymeric vector induces a favorable therapeutic effect, especially when in combination with oxaliplatin. Our study uncovers a novel function of AMPKα1 in redox regulation and identifies a promising therapeutic strategy for treatment of CRC.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo.
- Record: found
- Abstract: found
- Article: not found
Global Phosphoproteomic Analysis of Human Skeletal Muscle Reveals a Network of Exercise-Regulated Kinases and AMPK Substrates.
- Record: found
- Abstract: found
- Article: not found