- Record: found
- Abstract: found
- Article: found
Intravitreal anti-VEGF injections for treating wet age-related macular degeneration: a systematic review and meta-analysis
Abstract
Aims
Age-related macular degeneration (AMD) is the main cause of blindness. Anti-vascular endothelial growth factor is used to prevent further neovascularization due to wet AMD. The purpose of this systematic review was to investigate the effect and protocol of anti-vascular endothelial growth factor treatment on wet AMD.
Methods
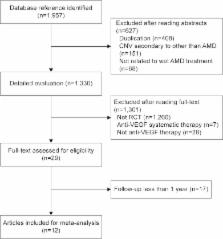
A comprehensive literature search was performed in PubMed, Embase, the Cochrane Library, CNKI, and reference lists. Meta-analysis was performed using Stata12.0 software, best corrected visual acuity (BCVA), retinal thickness, and lesion size were evaluated.
Results
Twelve randomized controlled trials spanning from 2010 to 2014 and involving 5,225 patients were included. A significant difference was observed between the intravitreal ranibizumab (IVR) group and the intravitreal bevacizumab group (standard mean difference =−0.14, 95% confidence interval [CI] =−0.23 to −0.05). No significant differences were observed in best corrected VA, retinal thickness, or lesion size between IVR and the intravitreal aflibercept group. Compared to monthly injection, IVR as-needed injections (PRN) can raise VA by 1.97 letters (weighted mean difference =1.97, 95% CI =0.14–3.794). Combination therapy of IVR and photodynamic therapy can significantly raise VA by 2.74 letters when combined with IVR monotherapy (weighted mean difference =2.74, 95% CI =0.26–5.21).
Conclusion
The superiority remains unclear between IVR and intravitreal bevacizumab in the treatment of neovascular AMD. Intravitreal aflibercept dosed every 2 months required fewer injection times, but produced similar efficacy as monthly IVR. IVR PRN could significantly increase VA. Combined with photodynamic therapy, IVR therapy could also increase VA effectively.
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Prevalence of age-related macular degeneration in the United States.
- Record: found
- Abstract: found
- Article: not found
Pegaptanib for neovascular age-related macular degeneration.
- Record: found
- Abstract: found
- Article: not found