- Record: found
- Abstract: found
- Article: found
Long non-coding RNA UCA1 promotes malignant phenotypes of renal cancer cells by modulating the miR-182-5p/DLL4 axis as a ceRNA
Read this article at
Abstract
Background
Accumulating literatures have indicated that long non-coding RNAs (lncRNAs) are potential biomarkers that play key roles in tumor development and progression. Urothelial cancer associated 1 (UCA1) is a novel lncRNA that acts as a potential biomarker and is involved in the development of cancers. However, the molecular mechanism of UCA1 in renal cancer is still needed to further explore.
Methods
The relative expression level of UCA1 was determined by Real-Time qPCR in a total of 88 patients with urothelial renal cancer and in different renal cancer cell lines. Loss-of-function experiments were performed to investigate the biological roles of UCA1 and miR-182-5p on renal cancer cell proliferation, migration, apoptosis and tumorigenicity. Comprehensive transcriptional analysis, dual-luciferase reporter assay and western blot etc. were performed to explore the molecular mechanisms underlying the functions of UCA1.
Results
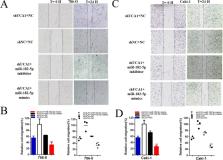
In this study, we found that UCA1 was significantly up-regulated in renal cancer. Moreover, increased UCA1 expression was positively correlated with differentiation and advanced TNM stage. Further experiments demonstrated that knockdown of UCA1 inhibited malignant phenotypes and Notch signal path of renal cancer cells, and miR-182-5p was reverse function as UCA1. UCA1 functioned as a miRNA sponge to positively regulate the expression of Delta-like ligand 4(DLL4) through sponging miR-182-5p and subsequently promoted malignant phenotypes of renal cancer cells, thus UCA1 playing an oncogenic role and miR-182-5p as an antioncogenic one in renal cancer pathogenesis.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Structural organization of the inactive X chromosome in the mouse.
- Record: found
- Abstract: found
- Article: not found
Feedback modulation of cholesterol metabolism by LeXis, a lipid-responsive non-coding RNA

- Record: found
- Abstract: found
- Article: found