- Record: found
- Abstract: found
- Article: found
AMPK antagonizes hepatic glucagon-stimulated cyclic AMP signalling via phosphorylation-induced activation of cyclic nucleotide phosphodiesterase 4B

Read this article at
Abstract
Biguanides such as metformin have previously been shown to antagonize hepatic glucagon-stimulated cyclic AMP (cAMP) signalling independently of AMP-activated protein kinase (AMPK) via direct inhibition of adenylate cyclase by AMP. Here we show that incubation of hepatocytes with the small-molecule AMPK activator 991 decreases glucagon-stimulated cAMP accumulation, cAMP-dependent protein kinase (PKA) activity and downstream PKA target phosphorylation. Moreover, incubation of hepatocytes with 991 increases the V max of cyclic nucleotide phosphodiesterase 4B (PDE4B) without affecting intracellular adenine nucleotide concentrations. The effects of 991 to decrease glucagon-stimulated cAMP concentrations and activate PDE4B are lost in hepatocytes deleted for both catalytic subunits of AMPK. PDE4B is phosphorylated by AMPK at three sites, and by site-directed mutagenesis, Ser304 phosphorylation is important for activation. In conclusion, we provide a new mechanism by which AMPK antagonizes hepatic glucagon signalling via phosphorylation-induced PDE4B activation.
Abstract
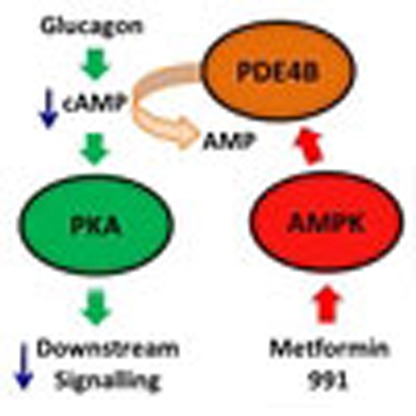 The diabetes drug Metformin decreases hepatic glucose production and activates AMP-activated
protein kinase (AMPK). Here the authors provide evidence that AMPK activation antagonizes
glucagon signalling by activating PDE4B, lowering cAMP levels and decreasing PKA activation.
The diabetes drug Metformin decreases hepatic glucose production and activates AMP-activated
protein kinase (AMPK). Here the authors provide evidence that AMPK activation antagonizes
glucagon signalling by activating PDE4B, lowering cAMP levels and decreasing PKA activation.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism.
- Record: found
- Abstract: found
- Article: not found
Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats.
- Record: found
- Abstract: found
- Article: not found