- Record: found
- Abstract: found
- Article: found
Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs)
Abstract
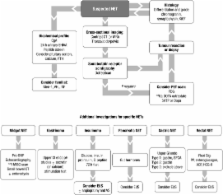
These guidelines update previous guidance published in 2005. They have been revised by a group who are members of the UK and Ireland Neuroendocrine Tumour Society with endorsement from the clinical committees of the British Society of Gastroenterology, the Society for Endocrinology, the Association of Surgeons of Great Britain and Ireland (and its Surgical Specialty Associations), the British Society of Gastrointestinal and Abdominal Radiology and others. The authorship represents leaders of the various groups in the UK and Ireland Neuroendocrine Tumour Society, but a large amount of work has been carried out by other specialists, many of whom attended a guidelines conference in May 2009. We have attempted to represent this work in the acknowledgements section. Over the past few years, there have been advances in the management of neuroendocrine tumours, which have included clearer characterisation, more specific and therapeutically relevant diagnosis, and improved treatments. However, there remain few randomised trials in the field and the disease is uncommon, hence all evidence must be considered weak in comparison with other more common cancers.
Related collections
Most cited references402
- Record: found
- Abstract: found
- Article: not found
The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology.
- Record: found
- Abstract: found
- Article: not found
Sunitinib malate for the treatment of pancreatic neuroendocrine tumors.
- Record: found
- Abstract: found
- Article: not found