- Record: found
- Abstract: found
- Article: found
Early Prognostic Utility of Gp210 Antibody-Positive Rate in Primary Biliary Cholangitis: A Meta-Analysis
Read this article at
Abstract
Background
The prevalence of primary biliary cholangitis (PBC), which is an autoimmune liver disease, has increased over time. PBC often leads to severe consequences, such as liver failure and death. Stratification tools using biochemical liver tests are needed to assess and predict the progression of this disease at the time of PBC diagnosis.
Methods
We searched PubMed, Cochrane Library, Web of Science, and Embase for studies focused on the relationship between positive rates of Gp210 antibodies and poor prognosis of PBC. The primary end point was the number of PBC patients with poor outcome in the Gp210 antibody (+) and Gp210 antibody (−) groups. The secondary end point was the basic serum level of alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin (TBIL), and IgM in the two groups. The age and number of female patients were also measured.
Results
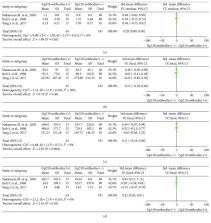
A total of 5 studies, comprising 737 patients, were included in this analysis. A positive rate of Gp210 antibodies was positively correlated with poor outcomes and with many types of progression in PBC, especially liver failure. Mortality was also higher in the Gp210 antibody (+) group. Furthermore, the serum levels of ALP and IgM were associated with the positive rate of Gp210 antibodies, while the serum levels of ALT and TBIL were not. The age and number of female patients were also not associated with the positive rate of Gp210 antibodies.
Conclusion
PBC-specific Gp120 antibodies are optimal predictors of PBC prognosis at the time of diagnosis. Some other liver function indicators, such as ALP and IgM, can be used as predictors to complement Gp210 antibodies to establish a stratification tool to predict the prognosis of PBC at the time of diagnosis.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis
- Record: found
- Abstract: not found
- Article: not found
Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases
- Record: found
- Abstract: found
- Article: not found