- Record: found
- Abstract: found
- Article: found
Cardiomyocytes Derived from Human CardiopoieticAmniotic Fluids
Read this article at
Abstract
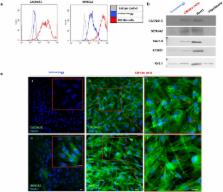
Human amniotic fluid (hAF) cells share characteristics of both embryonic and adult stem cells. They proliferate rapidly and can differentiate into cells of all embryonic germ layers but do not form teratomas. Embryoid-bodies obtained from hAF have cardiac differentiation potential, but terminal differentiation to cardiomyocytes (CMs) has not yet been described. Our purpose was to promote cardiac differentiation in hAFcells. Cells were exposed to inducing factors for up to 15 days. Only the subset of hAF cells expressing the multipotency markers SSEA4, OCT4 and CD90 ( CardiopoieticAF cells) responded to the differentiation process by increasing the expression of the cardiac transcription factors Nkx2.5 and GATA4, sarcomeric proteins (cTnT, α-MHC, α-SA), Connexin43 and atrial and ventricular markers. Furthermore, differentiated cells were positive for the calcium pumps CACNA1C and SERCA2a, with approximately 30% of CardiopoieticAF-derived CM-like cells responding to caffeine or adrenergic stimulation. Some spontaneous rare beating foci were also observed. In conclusion, we demonstrated that CardiopoieticAF cells might differentiate toward the cardiac lineage giving rise to CM-like cells characterized by several cardiac-specific molecular, structural, and functional properties.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
hESC-Derived Cardiomyocytes Electrically Couple and Suppress Arrhythmias in Injured Hearts
- Record: found
- Abstract: found
- Article: not found
Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies.
- Record: found
- Abstract: found
- Article: not found