- Record: found
- Abstract: found
- Article: found
Improved Brain Penetration and Antitumor Efficacy of Temozolomide by Inhibition of ABCB1 and ABCG2 1
Read this article at
Abstract
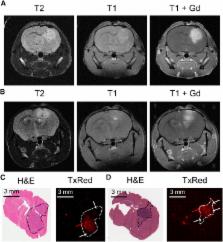
The anticancer drug temozolomide is the only drug with proven activity against high-grade gliomas and has therefore become a part of the standard treatment of these tumors. P-glycoprotein (P-gp; ABCB1) and breast cancer resistance protein (BCRP; ABCG2) are transport proteins, which are present at the blood-brain barrier and limit the brain uptake of substrate drugs. We have studied the effect of P-gp and BCRP on the pharmacokinetics and pharmacodynamics of temozolomide, making use of a comprehensive set of in vitro transport experiments and in vivo pharmacokinetic and antitumor efficacy experiments using wild-type, Abcg2 −/−, Abcb1a/b −/−, and Abcb1a/b;Abcg2 −/− mice. We here show that the combined deletion of Abcb1a/b and Abcg2 increases the brain penetration of temozolomide by 1.5-fold compared to wild-type controls ( P < .001) without changing the systemic drug exposure. Moreover, the same increase was achieved when temozolomide was given to wild-type mice in combination with the dual P-gp/BCRP inhibitor elacridar (GF120918). The antitumor efficacy of temozolomide against three different intracranial tumor models was significantly enhanced when Abcb1a/b and Abcg2 were genetically deficient or pharmacologically inhibited in recipient mice. These findings call for further clinical testing of temozolomide in combination with elacridar for the treatment of gliomas, as this offers the perspective of further improving the antitumor efficacy of this already active agent.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials.
- Record: found
- Abstract: found
- Article: not found
A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse
- Record: found
- Abstract: found
- Article: not found