- Record: found
- Abstract: found
- Article: not found
Arrhythmogenic mechanisms in the isolated perfused hypokalaemic murine heart
Read this article at
Abstract
Aim
Hypokalaemia is associated with a lethal form of ventricular tachycardia (VT), torsade de pointes, through pathophysiological mechanisms requiring clarification.
Methods
Left ventricular endocardial and epicardial monophasic action potentials were compared in isolated mouse hearts paced from the right ventricular epicardium perfused with hypokalaemic (3 and 4 m m [K +] o) solutions. Corresponding K + currents were compared in whole-cell patch-clamped epicardial and endocardial myocytes.
Results
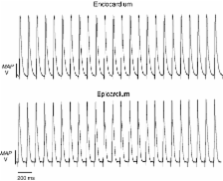
Hypokalaemia prolonged epicardial action potential durations (APD) from mean APD 90s of 37.2 ± 1.7 ms ( n = 7) to 58.4 ± 4.1 ms ( n =7) and 66.7 ± 2.1 ms ( n = 11) at 5.2, 4 and 3 m m [K +] o respectively. Endocardial APD 90s correspondingly increased from 51.6 ± 1.9 ms ( n = 7) to 62.8 ± 2.8 ms ( n = 7) and 62.9 ± 5.9 ms ( n = 11) giving reductions in endocardial–epicardial differences, ΔAPD 90, from 14.4 ± 2.6 to 4.4 ± 5.0 and −3.4 ± 6.0 ms respectively. Early afterdepolarizations (EADs) occurred in epicardia in three of seven spontaneously beating hearts at 4 m m [K +] o with triggered beats followed by episodes of non-sustained VT in nine of 11 preparations at 3 m m. Programmed electrical stimulation never induced arrhythmic events in preparations perfused with normokalemic solutions yet induced VT in two of seven and nine of 11 preparations at 4 and 3 m m [K +] o respectively. Early outward K + current correspondingly fell from 73.46 ± 8.45 to 61.16±6.14 pA/pF in isolated epicardial but not endocardial myocytes ( n = 9) (3 m m [K +] o).
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Pharmacology of cardiac potassium channels.
- Record: found
- Abstract: found
- Article: not found
