- Record: found
- Abstract: found
- Article: found
Regulation of the Spontaneous Augmentation of Na V1.9 in Mouse Dorsal Root Ganglion Neurons: Effect of PKA and PKC Pathways
Read this article at
Abstract
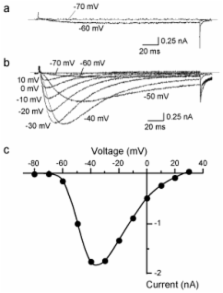
Sensory neurons in the dorsal root ganglion express two kinds of tetrodotoxin resistant (TTX-R) isoforms of voltage-gated sodium channels, Na V1.8 and Na V1.9. These isoforms play key roles in the pathophysiology of chronic pain. Of special interest is Na V1.9: our previous studies revealed a unique property of the Na V1.9 current, i.e., the Na V1.9 current shows a gradual and notable up-regulation of the peak amplitude during recording (“spontaneous augmentation of Na V1.9”). However, the mechanism underlying the spontaneous augmentation of Na V1.9 is still unclear. In this study, we examined the effects of protein kinases A and C (PKA and PKC), on the spontaneous augmentation of Na V1.9. The spontaneous augmentation of the Na V1.9 current was significantly suppressed by activation of PKA, whereas activation of PKA did not affect the voltage dependence of inactivation for the Na V1.9 current. On the contrary, the finding that activation of PKC can affect the voltage dependence of inactivation for Na V1.9 in the perforated patch recordings, where the augmentation does not occur, suggests that the effects of PMA are independent of the augmentation process. These results indicate that the spontaneous augmentation of Na V1.9 was regulated directly by PKA, and indirectly by PKC.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches.
- Record: found
- Abstract: found
- Article: not found
The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways.
- Record: found
- Abstract: found
- Article: not found