- Record: found
- Abstract: found
- Article: found
Dual roles of nitric oxide in the regulation of tumor cell response and resistance to photodynamic therapy
Read this article at
Abstract
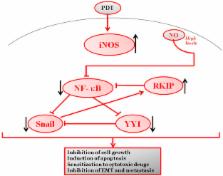
Photodynamic therapy (PDT) against cancer has gained attention due to the successful outcome in some cancers, particularly those on the skin. However, there have been limitations to PDT applications in deep cancers and, occasionally, PDT treatment resulted in tumor recurrence. A better understanding of the underlying molecular mechanisms of PDT-induced cytotoxicity and cytoprotection should facilitate the development of better approaches to inhibit the cytoprotective effects and also augment PDT-mediated cytotoxicity. PDT treatment results in the induction of iNOS/NO in both the tumor and the microenvironment. The role of NO in cytotoxicity and cytoprotection was examined. The findings revealed that NO mediates its effects by interfering with a dysregulated pro-survival/anti-apoptotic NF-κB/Snail/YY1/RKIP loop which is often expressed in cancer cells. The cytoprotective effect of PDT-induced NO was the result of low levels of NO that activates the pro-survival/anti-apoptotic NF-κB, Snail, and YY1 and inhibits the anti-survival/pro-apoptotic and metastasis suppressor RKIP. In contrast, PDT-induced high levels of NO result in the inhibition of NF-kB, Snail, and YY1 and the induction of RKIP, all of which result in significant anti-tumor cytotoxicity. The direct role of PDT-induced NO effects was corroborated by the use of the NO inhibitor, l-NAME, which reversed the PDT-mediated cytotoxic and cytoprotective effects. In addition, the combination of the NO donor, DETANONOate, and PDT potentiated the PDT-mediated cytotoxic effects. These findings revealed a new mechanism of PDT-induced NO effects and suggested the potential therapeutic application of the combination of NO donors/iNOS inducers and PDT in the treatment of various cancers. In addition, the study suggested that the combination of PDT with subtoxic cytotoxic drugs will result in significant synergy since NO has been shown to be a significant chemo-immunosensitizing agent to apoptosis.
Graphical abstract
Highlights
-
•
PDT-mediated cytotoxic and cytoprotective effects depend also by the induction of NO from tumor.
-
•
The PDT-induced NO modulates the dysregulated NF-kB/Snail/RKIP loop.
-
•
The direct role of NO induction by PDT was corroborated by the use of the NO inhibitor, l-NAME.
-
•
The combination of an NO donor and PDT resulted in a increased cytotoxic effect, in vitro and in vivo.
-
•
Novel potential therapeutic applications are proposed for the use of PDT combined with NO donors.
Related collections
Most cited references86
- Record: found
- Abstract: found
- Article: not found
The chemical biology of nitric oxide: implications in cellular signaling.
- Record: found
- Abstract: found
- Article: not found
Necrotic death as a cell fate.
- Record: found
- Abstract: found
- Article: not found
