- Record: found
- Abstract: found
- Article: found
Management of glucocorticoids-induced osteoporosis: role of teriparatide
Read this article at
Abstract
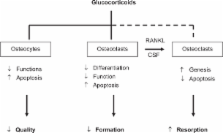
Glucocorticoids (GC)-induced osteoporosis (GIOP) is the most common cause of secondary osteoporosis, which leads to an increased fracture risk in patients. The normal bone turnover depends on a balance between osteoblasts and osteoclasts activity and GC can cause a rapid bone loss, decreasing bone formation and increasing bone resorption. The decreased bone formation is mainly due to the GC-induced apoptosis of both osteoblasts and osteocytes, while the increased bone resorption is due to the increased life-span of pre-existing osteoclasts. Bisphosphonates are clearly effective in preventing and treating GIOP but anabolic therapeutic strategies are the new promising therapeutic alternative. Experimental and clinical studies indicate that teriparatide, the active (1–34) parathyroid hormone (PTH) molecule, is efficacious for the treatment of GIOP, being able to induce an increase in bone mass in these patients. Intermittent administration of human PTH (1–34) stimulates bone formation by increasing osteoblast number. Additionally, human PTH (1–34) modulates the level and/or activity of locally produced growth factors and cytokines. Teriparatide has been demonstrated in several clinical studies to significantly decrease the incidence of fractures in patients affected by GIOP. It has recently received an indication for GIOP and its label indication has also been expanded.
Most cited references80
- Record: found
- Abstract: found
- Article: not found
Teriparatide or alendronate in glucocorticoid-induced osteoporosis.
- Record: found
- Abstract: found
- Article: not found