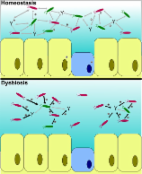
Indigenous microbiota play a critical role in the lives of their vertebrate hosts. In human and mouse models it is increasingly clear that innate and adaptive immunity develop in close concert with the commensal microbiome. Furthermore, several aspects of digestion and nutrient metabolism are governed by intestinal microbiota. Research on teleosts has responded relatively slowly to the introduction of massively parallel sequencing procedures in microbiomics. Nonetheless, progress has been made in biotic and gnotobiotic zebrafish models, defining a core microbiome and describing its role in development. However, microbiome research in other teleost species, especially those important from an aquaculture perspective, has been relatively slow. In this review, we examine progress in teleost microbiome research to date. We discuss teleost microbiomes in health and disease, microbiome ontogeny, prospects for successful microbiome manipulation (especially in an aquaculture setting) and attempt to identify important future research themes. We predict an explosion in research in this sector in line with the increasing global demand for fish protein, and the need to find sustainable approaches to improve aquaculture yield. The reduced cost and increasing ease of next generation sequencing technologies provides the technological backing, and the next 10 years will be an exciting time for teleost microbiome research.