- Record: found
- Abstract: found
- Article: found
The natriuretic peptides system in the pathophysiology of heart failure: from molecular basis to treatment
Read this article at
Abstract
This article overviews the natriuretic peptides (NPs) system, its role and the development of NP-based treatment of heart failure (HF).
Abstract
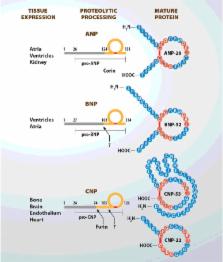
After its discovery in the early 1980s, the natriuretic peptide (NP) system has been extensively characterized and its potential influence in the development and progression of heart failure (HF) has been investigated. HF is a syndrome characterized by the activation of different neurohormonal systems, predominantly the renin–angiotensin (Ang)–aldosterone system (RAAS) and the sympathetic nervous system (SNS), but also the NP system. Pharmacological interventions have been developed to counteract the neuroendocrine dysregulation, through the down modulation of RAAS with ACE (Ang-converting enzyme) inhibitors, ARBs (Ang receptor blockers) and mineralcorticoid antagonists and of SNS with β-blockers. In the last years, growing attention has been paid to the NP system. In the present review, we have summarized the current knowledge on the NP system, focusing on its role in HF and we provide an overview of the pharmacological attempts to modulate NP in HF: from the negative results of the study with neprilysin (NEP) inhibitors, alone or associated with an ACE inhibitor and vasopeptidase inhibitors, to the most recently and extremely encouraging results obtained with the new pharmacological class of Ang receptor and NEP inhibitor, currently defined ARNI (Ang receptor NEP inhibitor). Indeed, this new class of drugs to manage HF, supported by the recent results and a vast clinical development programme, may prompt a conceptual shift in the treatment of HF, moving from the inhibition of RAAS and SNS to a more integrated target to rebalance neurohormonal dysregulation in HF.
Related collections
Most cited references190
- Record: found
- Abstract: found
- Article: not found
The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial.
- Record: found
- Abstract: found
- Article: not found
Effect of nesiritide in patients with acute decompensated heart failure.
- Record: found
- Abstract: found
- Article: not found