- Record: found
- Abstract: found
- Article: found
Emphysema: time to say farewell to therapeutic nihilism
editorial
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
It is an interesting time for the management of emphysema. In this condition, destruction
of lung parenchyma associated with reduced elastic recoil and dynamic airways closure
produce gas trapping and increased operating lung volumes, leading to breathlessness
and exercise limitation. It has historically been defined as an irreversible process,
which has led to a degree of therapeutic nihilism. One manifestation of this has been
the curious neglect of lung volume reduction surgery (LVRS). Clinical guidelines,1
reflecting trial evidence,2 recommend consideration of LVRS in selected patients with
upper lobe predominant emphysema and poor exercise capacity, the phenotype where surgery
has been shown to produce a survival benefit. Modern surgical techniques, unilateral
treatment and improved postoperative care and patient selection mean that LVRS is
also associated with lower morbidity and mortality than data published at the turn
of the century had suggested,3
4 with one recent case series reporting zero 90-day mortality following unilateral
surgery.5
Nevertheless, little effort seems to be going into identifying this patient population
and LVRS remains vastly underused with just 90 procedures taking place in the UK in
2010–2011. A partial explanation for this may be found in a recent survey of British
Thoracic Society members that revealed that a significant proportion overestimated
the morbidity and mortality associated with LVRS.6 Only 30% had access to a dedicated
chronic obstructive pulmonary disease (COPD) multidisciplinary meeting to review patients,
and there was no consensus as to the correct strategy to adopt to identify appropriate
patients.
Over the last decade, bronchoscopic approaches for lung volume reduction in emphysema
have proliferated. These include one-way endobronchial valves to induce lobar collapse,7–10
airway bypass approaches to create low-resistance extra-anatomical pathways that allow
trapped gas to escape,11
12 lung volume reduction coils (LVRC) that re-tension the lung preventing dynamic
airway collapse13 and techniques intended to reduce lung volumes by scarring either
through bronchial thermal vapour ablation (‘steam’)14 or the use of biological agents.15
16 These approaches offer the potential to achieve lung volume reduction, improving
symptoms and even prolonging survival8 while avoiding the problems inherent in an
invasive surgical intervention. However, for each approach issues around the magnitude
and duration of effect, optimum patient selection, safety profile and cost need to
be addressed through properly conducted clinical trials with robust endpoints.
Deslee and colleagues present 6-month and 12-month data from a multicentre, single-arm
study of staged, bilateral LVRC treatment in patients with severe emphysema.17 They
report improvements in St George's Respiratory Questionnaire (SGRQ) scores of −11.1±13.3
points 12 months following therapy as compared with baseline, with persistent benefits
in 6 minute walking distance (6MWD), forced expiratory flow in the 1st second (FEV1)
and residual volume (RV) exceeding accepted minimal clinically important differences.
These data are encouraging and add to existing data from short-term controlled trials,13
but the limitations of this study merit some consideration when considering the general
issues for bronchoscopic therapies outlined above. It was uncontrolled and unblinded,
with only roughly half the cohort followed up beyond 6 months and the primary outcome
was a quality of life score. This is questionable in a single-arm study without a
sham procedure, given the powerful placebo effect seen with this sort of intervention.
Nevertheless, the sustained improvement in lung volumes seems to indicate a persistent
physiological effect. Longer-term follow-up in randomised controlled studies will
be needed to be able to comment definitively on sustained benefits of LVRC. The RENEW
trial (clinicaltrials.gov NCT01608490), which is currently underway with a primary
endpoint of 6MWD 12 months post-recruitment will address this.
The authors do not clarify what the distribution of recruitment among the 11 participating
centres was or their prior experience with the technique. Two patients recruited for
bilateral coil treatment were then thought to be unsuitable for contralateral treatment
on a ‘second look’ at their imaging. In addition, the trial was meant to enrol only
patients with heterogeneous disease, yet 13 and 17 of 33 patients reaching 12 months’
follow-up were deemed ‘homogeneous’ on visual and computerised scoring, respectively.
The authors propose that homogeneous emphysema responds to treatment with LVRC to
a similar extent to heterogeneous disease, but this must be considered hypothesis
generating only and to some extent seems to reflect a failure in the application of
the initial protocol. The problem of recruiting patients for lung volume reduction
trials who on post hoc analysis do not fulfil entry criteria is not unique to this
study.7
11
Safety is another key issue. It has been assumed that bronchoscopic lung volume reduction
is safer and cheaper than LVRS. Pneumothorax occurred within 30 days in 4 of 155 procedures
(3.5%), with a per patient rate of 11.7% (7/60) during the follow-up period. Pneumothorax
following bronchoscopic lung volume reduction procedures can be delayed and can be
fatal. This means that as well as formal safety criteria around lung function and
exercise capacity a general clinical assessment of the patient's likely ability to
cope with this complication needs to be made. In practice, relatively few people judged
‘too unwell for LVRS’ may be eligible for a bronchoscopic approach. All patients treated
as part of this trial had general anaesthesia in an operating theatre, but it should
be noted that these procedures can be performed safely in the bronchoscopy suite under
sedation.13
A review article written a decade ago posed the question ‘Endobronchial lung volume
reduction, a myth, or a marvel?’18 The proliferation of techniques and publications
mean that these approaches can no longer be considered mythical, but nor any longer
are they ‘a marvel’. Rather, they must be considered as techniques with a developing
evidence base and varying response rates and complications where a case must be made
that they represent good value, considered in terms of the resources employed and
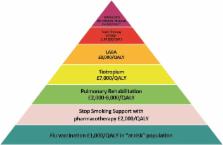
the health outcomes obtained. The London Respiratory Network has produced a value
pyramid for COPD interventions19 (figure 1), but it remains to be established where
each bronchoscopic approach will sit. LVRS was estimated as costing $40,000 per quality
adjusted life year (QALY) based on the US National Emphysema Treatment Trial (NETT),20
but the likely true figure is considerably lower, given improvements in technique
with reduced mortality, morbidity and length of stay.5
21 The cost of LVRCs is at present high with the list price of the devices themselves
significantly exceeding the national LVRS tariff in the UK.
Figure 1
The pyramid of value for COPD interventions developed by the London Respiratory Network
with The London School of Economics (modified from19) gives estimates of cost per
quality adjusted life year gained. LABA long-acting β2 agonist; QALY, quality adjusted
life year.
As with LVRS, the response rates for bronchoscopic techniques are crucially dependent
on appropriate patient selection, with different criteria for different devices. This
takes us back to the need to develop an multi-disciplinary team (MDT) approach for
emphysema5
21 (table 1). Given trial data indicating improved survival, a failure to offer LVRS
to appropriate patients with COPD and by extension a failure to make the effort to
identify them seems to us to border on negligence. The development of a network of
emphysema MDTs should also facilitate the more rapid delivery of trials to investigate
the efficacy of experimental treatments and ensure that appropriate criteria are used
to select individuals for more established interventions such as endobronchial valves
to ensure a high responder rate and the best value for the healthcare system.
Table 1
Approach to selecting patients with emphysema for a possible lung volume reduction
procedure
General criteria when considering a lung volume reduction procedure▸ Significantly
reduced exercise capacity.▸ Lung function impairment with significant hyperinflation.▸
Sufficiently well to cope with surgery.▸ Prepared to accept some procedural risk (requires
clinicians to be able to communicate this accurately).▸ There is a ‘window of opportunity’
for intervention. In ‘end-stage’ patients, it may be too late to intervene safely.
Considerations
Criteria
▸ Are they too well to consider intervention?
▸ Lung function, exercise capacity, prognosis, Medical Research Council dyspnoea score
<3
▸ Are they too unwell for intervention to be safe?
▸ Lung function, frailty, exercise capacity <100 m, oxygen dependence
▸ Is treatment optimal?
▸ Smoking cessation, pulmonary rehabilitation, flu vaccination, inhaled and oral medication
▸ Is their lung function likely to rule out a procedure on safety grounds?
▸ All three of FEV1, TLco and Kco <20% predicted
▸ Do they have comorbidities that limit likely benefit or increase risk?
▸ For example, pulmonary hypertension, unstable cardiac disease, malignancy, cerebrovascular
disease. Ongoing smoking (possibility of intervention may help to promote quit attempts)
▸ Have they ever had a CT thorax and if so has it been reported in terms of emphysema
pattern?
▸ Review existing CT's or obtain a CT if a potential candidate as above
Review CT and lung function in multi-disciplinary teams including respiratory physician,
radiologist, thoracic surgeonFurther investigations including echocardiogram, lung
perfusion scan and a formal field exercise test (shuttle walk or 6 minute walk test)
may be indicated.
Related collections
Most cited references14
- Record: found
- Abstract: found
- Article: not found
A randomized study of endobronchial valves for advanced emphysema.
Kevin L Kovitz, Geoffrey McLennan, … (2010)
- Record: found
- Abstract: found
- Article: not found
The National Emphysema Treatment Trial (NETT) Part II: Lessons learned about lung volume reduction surgery.
L Sternberg, Alvaro L F Martinez, Gerard J. Criner … (2011)
- Record: found
- Abstract: found
- Article: not found
Bronchoscopic lung-volume reduction with Exhale airway stents for emphysema (EASE trial): randomised, sham-controlled, multicentre trial.
PL Shah, D-J Slebos, PFG Cardoso … (2011)