- Record: found
- Abstract: found
- Article: found
Formulation and bioequivalence studies of choline alfoscerate tablet comparing with soft gelatin capsule in healthy male volunteers
Abstract
Purpose
The aim of this study was to develop a tablet formulation of choline alfoscerate and to assess its bioequivalence by comparing its pharmacokinetic parameters with those of a commercially available soft gelatin capsule (Gliatilin ®) in healthy Korean male volunteers.
Materials and methods
Film-coated tablet formulation was optimized to control the hygroscopicity of choline alfoscerate. Bioequivalence study was performed under fasted condition with a randomized, single-dose, two-period crossover design. Subjects were orally treated with 1,200 mg of test or reference choline alfoscerate (400 mg × three doses) formulation. Blood samples were collected up to 12 hours the day before dosing to correct the baseline level of choline and 12 hours after dosing to obtain drug absorption profile. Pharmacokinetic parameters were determined after analyzing plasma concentration of choline by using LC–MS/MS.
Results
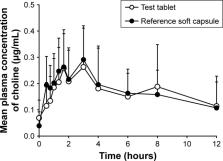
Hygroscopicity of choline alfoscerate was successfully controlled by adding suitable amount of Neusilin ® (magnesium aluminometasilicate) in the film-coated tablet. Stability of the tablet formulation was also confirmed under the accelerated condition for 3 months. Bioequivalence study showed that the mean area under the plasma concentration–time curve from time 0 to infinity of test tablet and reference soft capsule was 3.428±2.170 and 3.305±1.803 µg⋅h/mL, respectively; the mean C max was 0.365±0.158 and 0.380±0.108 µg/mL, respectively; and the mean T max was 3.51±2.57 and 3.85±3.19 hours, respectively. The 90% CIs for geometric mean ratios of test to reference formulation for AUC 0–t and C max were 84.51%–111.98% and 83.31%–104.10%, respectively, and satisfied the EMA regulatory criteria for bioequivalence.
Conclusion
Pharmacokinetic parameters including the C max and AUC 0–t determined after oral administration of the two formulations in healthy Korean male volunteers showed that the differences between the formulations (tablet vs soft capsule) were not significant for bioequivalence. Both formulations were well tolerated, with no serious adverse events reported.
Most cited references14
- Record: found
- Abstract: found
- Article: not found
Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry.
- Record: found
- Abstract: found
- Article: not found