- Record: found
- Abstract: found
- Article: found
TNK2 preserves epidermal growth factor receptor expression on the cell surface and enhances migration and invasion of human breast cancer cells
Read this article at
Abstract
Introduction
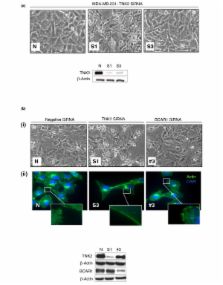
Amplification of the TNK2 gene in primary tumours correlates with poor prognosis. In accordance, TNK2 overexpression was shown to promote invasion of cancer cells – but the mechanism by which TNK2 mediates these effects is unresolved. TNK2 was suggested to regulate Cdc42-driven migration by activation of breast cancer antioestrogen resistance 1 (BCAR1); however, distinct from this effect is evidence for a role of TNK2 in the regulation of epidermal growth factor receptor (EGFR) endocytosis and degradation. In the present study we sought to investigate whether negative targeting of TNK2 by siRNA could be used to inhibit cancer cell invasion, to establish the contribution of its effect on the EGFR and to consequently attempt to resolve the issue of TNK2's mechanism of action.
Methods
We used siRNA to knockdown expression of TNK2 and its proposed effector BCAR1 in order to analyse the effect of this knockdown on cancer cell behaviour in vitro. We examined morphological changes using phase-contrast microscopy and immunohistochemistry. Functional parameters examined included apoptosis, proliferation, migration and invasion. We also performed flow cytometry analysis to examine EGFR cell surface expression and carried out western blot to examine the total EGFR levels.
Results
We observed that targeting of TNK2 by siRNA in breast cancer cells resulted in distinct morphological changes characterised by a stellate appearance and an absence of protrusions at membrane edges. These changes were not recapitulated upon siRNA targeting of BCAR1. We thus hypothesised that a component of the effects induced by TNK2 may be independent of BCAR1. Consistent with the idea of an alternative mechanism for TNK2, we observed that TNK2 associates with activated EGFR in breast cancer cells in a TNK2-kinase-independent manner. Furthermore, we demonstrated that TNK2 functions to maintain EGFRs on the cell surface. We could demonstrate that the main functional effect of activating these surface EGFRs in breast cancer cells is stimulation of migration. In accordance, TNK2 silencing by siRNA led to a significant reduction in cell surface EGFR and to a concomitant decrease in the migratory and invasive capacity of breast cancer cells.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer.
- Record: found
- Abstract: found
- Article: not found
Rho GTPases: signaling, migration, and invasion.
- Record: found
- Abstract: found
- Article: not found