- Record: found
- Abstract: found
- Article: found
The rise of multiple imputation: a review of the reporting and implementation of the method in medical research
Read this article at
Abstract
Background
Missing data are common in medical research, which can lead to a loss in statistical power and potentially biased results if not handled appropriately. Multiple imputation (MI) is a statistical method, widely adopted in practice, for dealing with missing data. Many academic journals now emphasise the importance of reporting information regarding missing data and proposed guidelines for documenting the application of MI have been published. This review evaluated the reporting of missing data, the application of MI including the details provided regarding the imputation model, and the frequency of sensitivity analyses within the MI framework in medical research articles.
Methods
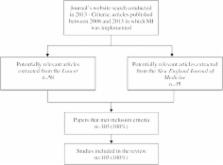
A systematic review of articles published in the Lancet and New England Journal of Medicine between January 2008 and December 2013 in which MI was implemented was carried out.
Results
We identified 103 papers that used MI, with the number of papers increasing from 11 in 2008 to 26 in 2013. Nearly half of the papers specified the proportion of complete cases or the proportion with missing data by each variable. In the majority of the articles (86%) the imputed variables were specified. Of the 38 papers (37%) that stated the method of imputation, 20 used chained equations, 8 used multivariate normal imputation, and 10 used alternative methods. Very few articles (9%) detailed how they handled non-normally distributed variables during imputation. Thirty-nine papers (38%) stated the variables included in the imputation model. Less than half of the papers (46%) reported the number of imputations, and only two papers compared the distribution of imputed and observed data. Sixty-six papers presented the results from MI as a secondary analysis. Only three articles carried out a sensitivity analysis following MI to assess departures from the missing at random assumption, with details of the sensitivity analyses only provided by one article.
Conclusions
This review outlined deficiencies in the documenting of missing data and the details provided about imputation. Furthermore, only a few articles performed sensitivity analyses following MI even though this is strongly recommended in guidelines. Authors are encouraged to follow the available guidelines and provide information on missing data and the imputation process.
Related collections
Most cited references80
- Record: found
- Abstract: found
- Article: not found
Missing data: our view of the state of the art.
- Record: found
- Abstract: found
- Article: not found
Ranibizumab and bevacizumab for neovascular age-related macular degeneration.
- Record: found
- Abstract: found
- Article: not found