- Record: found
- Abstract: found
- Article: found
Antimicrobial peptides in frog poisons constitute a molecular toxin delivery system against predators
Read this article at
Abstract
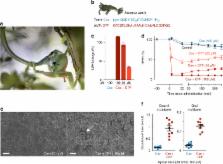
Animals using toxic peptides and proteins for predation or defense typically depend on specialized morphological structures, like fangs, spines, or a stinger, for effective intoxication. Here we show that amphibian poisons instead incorporate their own molecular system for toxin delivery to attacking predators. Skin-secreted peptides, generally considered part of the amphibian immune system, permeabilize oral epithelial tissue and enable fast access of cosecreted toxins to the predator’s bloodstream and organs. This absorption-enhancing system exists in at least three distantly related frog lineages and is likely to be a widespread adaptation, determining the outcome of predator–prey encounters in hundreds of species.
Abstract
To avoid being eaten, poisonous prey animals must rely on fast passage of toxins across a predator’s oral tissue, a major barrier to large molecules. Here, Raaymakers et al. show that antimicrobial peptides co secreted with frog toxins enhance intoxication of a snake predator by permeabilizing oral cell layers.
Related collections
Most cited references51
- Record: found
- Abstract: found
- Article: not found
Approaches for enhancing oral bioavailability of peptides and proteins.
- Record: found
- Abstract: found
- Article: found
Three Valuable Peptides from Bee and Wasp Venoms for Therapeutic and Biotechnological Use: Melittin, Apamin and Mastoparan
- Record: found
- Abstract: not found
- Article: not found