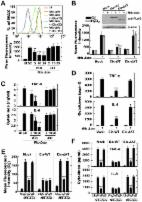
Introduction Mycobacterium tuberculosis (Mtb) is an intracellular pathogen that can survive and even multiply within host macrophages [1], [2]. Mtb can persist within phagosomes by interfering with intracellular membrane trafficking and by arresting phagosome maturation in infected host cells [3]. Pathogenic mycobacteria have developed several strategies for surviving and growing under nutrient-limited conditions [4]. Autophagy, or the removal of aged organelles, plays a central role in regulating important cellular functions [5], [6] and aids in innate and adaptive immune defense against Mtb and other intracellular pathogens [5], [7]–[9]. Physiological or pharmacological induction of autophagy in macrophages results in increased co-localization of mycobacterial phagosomes and the autophagy effector LC3, and the fusion of the former with lysosomes, which overcomes the blockade of membrane trafficking and increased bactericidal activity [7]. Although autophagy plays key roles in host innate and adaptive immune defenses, it can, under certain circumstances, result in type II programmed cell death [10], [11]. Autophagic processes are activated in response to cellular stresses, such as oxidative stress, and can influence several types of cell death, including autophagy-related cell death [12]. Recently, we showed that the mycobacterial BCG cell wall triggers autophagy-induced cell death in radiosensitized colon cancer cells [13]. Additionally, several viral gene products may be involved in autophagy-induced cell death [14]. However, the genetic basis for mycobacterial induction of autophagy, and its implications for host cell viability, remain to be elucidated. The “enhanced intracellular survival” (eis) gene and its protein product, Eis, a unique protein of 42 kDa, of Mtb H37Rv enhance the survival of the saprophytic M. smegmatis during repeated passage through the human macrophage-like cell line U-937 [15]. Bioinformatic analyses showed that Eis is a member of the GCN5-related family of N-acetyltransferases [16]. Recent studies have revealed that kanamycin resistance is associated with eis promoter mutations that increase Eis transcript and protein levels [17]. Additionally, regulation of eis expression by SigA enhanced intracellular growth of the W-Beijing Mtb strain in monocytic cells [18]. Moreover, Eis inhibited the proliferation of mitogen-activated T cells and, by blocking the phosphorylation of extracellular signal-regulated kinase (ERK), reduced the production of tumor necrosis factor (TNF)-α and interleukin (IL)-4 [19]. Despite being implicated in host-pathogen interactions during Mtb infection, the precise role of Eis in innate immune regulation remains to be determined. In an effort to gain further insight into the role of Eis in host responses, we examined autophagy, inflammatory cytokine production, and reactive oxygen species (ROS) generation in macrophages infected with wild-type (Mtb-WT), eis-deletion (Mtb-Δeis), or complemented (Mtb-c-eis) Mtb strains. Infection with Mtb-Δeis significantly increased autophagy, inflammatory responses, and ROS generation in macrophages. NADPH oxidase (NOX) and mitochondria were found to be the major sources of ROS, which contributed to the induction of autophagy and inflammatory responses in Mtb-Δeis-infected cells. Increased and excessive activation of autophagy in macrophages infected with Mtb-Δeis had no effect on antimicrobial responses, but stimulated caspase-independent cell death (CICD). Mtb-Δeis-induced host cell death was regulated by autophagic pathways and influenced by c-Jun N-terminal kinase (JNK)-dependent ROS generation. Furthermore, we show that the N-acetyltransferase domain of Eis is responsible for its modulation of ROS generation and proinflammatory responses in macrophages. Results Mycobacterium tuberculosis Eis Inhibits Autophagy in Macrophages Previous studies identified a role for the eis gene in enhancing the survival of mycobacteria in human monocytic cells [15]. However, the role of eis in autophagy activation in macrophages, which plays a key role in defense and cellular homeostasis [5], is not fully understood. We first infected bone marrow-derived macrophages (BMDMs) with the Mtb-WT, Mtb-Δeis, and Mtb-c-eis strains of Mtb H37Rv and examined the kinetics of autophagosome formation by immunostaining for LC3. As shown in Figure 1A, in BMDMs infected with Mtb-Δeis we observed the recruitment of endogenous LC3 in punctate structures the formation of which peaked 24 h after infection, before decreasing substantially by 48 h post-infection (Fig. 1A, right). In contrast, autophagosome formation was not increased in BMDMs infected with Mtb-WT or Mtb-c-eis (Fig. 1A). Additionally, RAW 264.7 macrophages transfected with green fluorescent protein (GFP) fused to the autophagosome protein LC3 (GFP-LC3) [20] showed a significant increase in GFP-LC3 puncta formation when infected with Mtb-Δeis at a multiplicity of infection (MOI) of 10 (over levels in cells infected with Mtb-WT or Mtb-c-eis at the same bacterial load; Fig. S1A). Moreover, Mtb-Δeis-induced formation of LC3 punctae in BMDMs (Fig. 1B) and RAW 264.7 cells (Fig. S1B) was abrogated by treatment for 4 h with 3-methyladenine (3-MA), a classical inhibitor of autophagy [21]. 10.1371/journal.ppat.1001230.g001 Figure 1 Mtb Eis modulates autophagy in macrophages. (A) BMDMs were infected with Mtb-WT, Mtb-Δeis, or Mtb-c-eis (MOI = 10) for 4 h (as described in the Materials and Methods), and then incubated for 24 h (left) or the indicated periods of time (right). Cells were fixed, stained with DAPI to visualize nuclei (blue), and immunolabeled with an anti-LC3 antibody. Primary antibody was detected using an Alexa Fluor 488-conjugated goat anti-rabbit IgG (green). Left: representative immunofluorescence images of LC3 punctae; right: quantification of data (LC3-punctated cells were counted manually). ***p 99%) were removed, as determined through staining with auramine-rhodamine (Merck, Darmstadt, Germany). After washing, the cells were incubated in fresh medium for a further 3 days. They were then lysed in autoclaved distilled water to allow intracellular bacteria to be collected [8]. The lysates were then re-suspended and sonicated for 5 min in a preheated 37°C water bath sonicator (Elma, Singen, Germany). Aliquots of the resulting sonicates were serially diluted in 7H9 broth, plated separately on 7H10 agar plates, and incubated at 37°C in 5% CO2 for 12 d. Colony counting was then performed in triplicate. Mice were challenged by aerosol exposure with Mtb-WT, Mtb-Δeis, or Mtb-c-eis using an inhalation device (Glas-Col, Terre Haute, IN, USA) calibrated to deliver approximately 50 bacteria into the lungs. Five mice per group were sacrificed at 4 weeks post-challenge, and bacteria in lung and spleen homogenates were counted. Numbers of viable bacteria in lung/spleen were determined by plating serial dilutions of whole organ homogenates on Middlebrook 7H11 agar (Difco, Detroit, MI, USA). Colonies were counted after 3–4 weeks of incubation at 37°C. Reagents, DNA, Abs, and Transfection DPI (a NOX inhibitor), NAC (an antioxidant), catalase, and z-VAD-fmk (a pan-caspase inhibitor) were purchased from Calbiochem (San Diego, CA, USA). 3-MA, tiron (a commercial deflocculant), and DAPI were purchased from Sigma. DMSO (Sigma) was added to cultures at a concentration of 0.1% (v/v) as a solvent control (SC). The plasmid that encoded EGFP-LC3 [20] was a gift from Tamotsu Yoshimori (Osaka University, Japan). pCMV-Eis-WT and pCMV-Eis-ΔAT constructs were created by subcloning the whole eis gene (WT), or an acetyltransferase domain-deletion mutant (lacking the sequence encoding residues 61–137 of the 402-amino-acid Eis protein) from pET21a. This was achieved by cutting at the BamHI and SacI restriction sites and then ligating the resulting inserts into the pCMV-Tag1 mammalian expression vector (Stratagene Co., USA). Anti-LC3 antibodies (Abs) used for Western blotting and immunofluorescence analysis were purchased from Novus Biologicals and MBL International (Woburn, MA, USA), respectively. Anti-rabbit IgG-Alexa488 and IgG-TRITC, and anti-mouse IgG-Cy2, were purchased from Jackson Immunoresearch (West Grove, PA, USA). siRNAs specific for mBeclin 1 (sc-29798), mAtg5 (sc-41446), and mJNK1 (sc-29381), each a pool of five target-specific 19–25 nt siRNAs, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Cells were transfected with plasmids and/or siRNAs using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. Measurement of ROS Production Intracellular ROS levels were measured by DCFH-DA and DHE assays as described previously [61]. Briefly, BMDMs were differentiated in culture dishes and infected with bacterial strains (MOI = 10) for 30 min. Cells were then incubated with either DCFH-DA (5 µM) or DHE (10 µM; Molecular Probes) for 30 min at 37°C in 5% CO2 and then washed with Krebs-Hepes buffer (for DHE staining) or HBSS (for DCFH-DA staining). Total intracellular levels of ROS were determined by FACS analyses of the oxidative conversion of cell-permeable DCFH-DA (Molecular Probes) to fluorescent DHE (Molecular Probes), using the FACSCanto II system (Becton Dickinson, San Jose, CA, USA). A mitochondrion-specific hydroethidine-derivative fluorescent dye (MitoSOX; M36008; Calbiochem) was used to determine relative mitochondrial O2 − levels in BMDMs. Cells were incubated for 30 min in PBS containing 5 µM MitoSOX. They were then washed twice and analyzed using the FACSCanto II system. All FACS data were collected using 50,000 to 100,000 cells and analyzed using FlowJo software (Tree Star, Ashland, OR, USA). Cell Viability and Apoptosis Assays Cell viability was assessed by PI staining and then examined by fluorescence microscopy or flow cytometric analysis. Trypan blue-stained cells were counted using a ViCell counter (Beckman Coulter, Fullerton, CA, USA). Apoptosis was examined by TdT-mediated dUTP Nick-End Labeling (TUNEL; Promega), according to the manufacturer's instructions. Labeled cells were examined under a laser-scanning confocal microscope (model LSM 510; Zeiss). Each condition was assayed in triplicate, and at least 200 cells per well were counted. To analyze in vivo cell death, single-cell suspensions were prepared in RPMI 1640 medium by passing cell populations through a nylon mesh with 50 µm pores and were subjected to further analysis. Western Blotting, RT-PCR and ELISA Treated BMDMs were processed for analysis by sandwich ELISA, Western blotting, and RT-PCR as described previously [2]. For Western blot analysis, primary Abs were diluted 1∶1000. Membranes were developed using a chemiluminescent reagent (ECL; Pharmacia-Amersham, Freiburg, Germany) and subsequently exposed to film (Pharmacia-Amersham). Supernatant TNF-α and IL-6 levels were measured by sandwich ELISA using Duoset Ab pairs (Pharmingen, San Diego, CA, USA) [2]. To provide RNA for RT-PCR analysis, paraffin-embedded tissue sections were first deparaffinized in octane [62]. After vigorous vortexing, 150 µL of methanol were added. Samples were vortexed again and the tissue was pelleted by centrifugation (10,000×g, 2 min). Supernatants were removed, and the remaining tissue was vacuum-dried for 20 min. Next, pellets were resuspended in digestion buffer (20 mM Tris-HCl, pH 7.6, 0.5% N-laurylsarcosine, 1 M guanidine thiocyanate, 25 mM 2-mercaptoethanol) containing proteinase K (5 mg/mL; Sigma). After overnight digestion at 55°C, RNA was extracted using TRIzol (Invitrogen) according to the manufacturer's instructions. For quantitative RT-PCR analysis was performed by using SYBR Green (Molecular Probes) PCR core reagents (Applied Biosystems), and transcript levels were quantified by using an ABI 7900 Sequence Detection System (Applied Biosystems). The mean value of triplicate reactions was normalized against the mean value of β-actin. Primers were used at 400 nM. Autophagy Analysis Autophagosome formation was measured by LC3 punctate staining, as described previously [8]. To quantitate autophagy, we used fluorescence microscopy to count the percentages of GFP-LC3-positive autophagic vacuoles in transfected cells or the numbers of endogenous LC3 punctate dots in primary cells. Each condition was assayed in triplicate, and at least 200 cells per well were counted. LC3 conjugation was evaluated by Western blot analysis using an antibody raised to LC3-I/II. Transmission Electron Microscopy Infected and stimulated RAW 264.7 macrophages were washed with PBS and then fixed with 3% formaldehyde, 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) for 1 h. They were then post-fixed in 1% osmium tetroxide, 0.5% potassium ferricyanide in cacodylate buffer for 1 h; embedded in straight resin; and cured at 80°C for 24 h. Ultrathin sections (70–80 nm), cut using an ultramicrotome (RMC MT6000-XL), were stained with uranyl acetate and lead citrate and examined using a Tecnai G2 Spirit Twin transmission electron microscope (FEI Company, USA) and a JEM ARM 1300S High Voltage electron microscope (JEOL, Japan). Immunofluorescence Immunofluorescence analysis was performed as described previously [8]. Briefly, cells were fixed with 4% paraformaldehyde in PBS at 4°C for 10 min and permeabilized with 0.01% Triton X-100 in PBS for 10 min. Cultures were then stained for 2 h at room temperature with primary antibodies, including rabbit anti-mouse LC3 (1∶400; MBL International). After washing, to remove excess primary antibody, cultures were then incubated for 1 h at room temperature with an anti-rabbit IgG-Alexa488 secondary antibody (Jackson Immunoresearch). Nuclei were stained by incubation with DAPI for 5 min. Slides were examined using a laser-scanning confocal microscope (model LSM 510; Zeiss). Statistical Analyses Data obtained from independent experiments (presented as mean±SD) were analyzed by the paired Student's t-test with Bonferroni correction or analysis of variance (for multiple comparisons). A p value<0.05 was deemed to indicate statistical significance. Accession Numbers The GenBank accession number for the eis gene is AF144099. Supporting Information Figure S1 Autophagic vesicles are increased in macrophages infected with Mtb-Δeis, but not in cells infected with Mtb-WT or Mtb-c-eis. (A and B) Formation of GFP-LC3 vacuoles (dots) was determined in RAW 264.7 cells transfected with GFP-LC3 cDNA. Transfected cells were infected with Mtb-WT, Mtb-Δeis, or Mtb-c-eis (MOI = 10) for 24 h (A) or Mtb-Δeis (MOI = 10) for 24 h in the presence or absence of 3-MA (B). Top, representative immunofluorescence images; bottom, percentage of GFP-LC3 cells with punctae. (C) Co-localization of autophagosomes (endogenous LC3, red) and lysosomes (lamp-1, green) was increased in Mtb-Δeis-infected BMDMs. Data are representative of three separate experiments. Scale bars: 10 µm. (D) BMDMs were infected with Mtb-Δeis (MOI = 10) for 24 h in the presence or absence of 3-MA (10 mM) or Baf-A1 (100 nM). Quantitation of the percentages of cells with LC3 punctae. Each condition was assayed in triplicate, and at least 250 cells per well were counted. ***p<0.001, vs. Mtb-WT-infected condition (A); SC (B and D). UI, uninfected; SC, solvent control (0.1% distilled water (B), 0.1% DMSO (D)). (0.66 MB TIF) Click here for additional data file. Figure S2 Activation of autophagy negatively impacts the secretion of proinflammatory cytokines by Mtb-Δeis-infected macrophages. RAW 264.7 cells transfected with siRNAs specific for Beclin-1 (siBec-1) or Atg5 (siAtg5) were infected with Mtb-WT, Mtb-Δeis, or Mtb-c-eis (MOI = 10) for 24 h. Supernatants were assessed by ELISA for levels of TNF-α and IL-6. Data are presented as the mean±SD of five experiments. ***p<0.001, vs. Mtb-WT-infected condition. UI, uninfected. (0.12 MB TIF) Click here for additional data file. Figure S3 Reactive nitrogen species are not involved in the elevation of ROS generation in Mtb-Δeis-infected macrophages. BMDMs were infected with Mtb-Δeis (MOI = 10) in the presence or absence of L-NAME (0.1, 1, 5 mM) or L-NMMA (0.1, 1, 5 mM). Cells were stained with DHE (for superoxide) or DCFH-DA (for H2O2) and subjected to flow cytometry analysis. Data represent densitometric analyses (mean±SD) of three separate experiments. UI, uninfected; SC, solvent control. (0.07 MB TIF) Click here for additional data file. Figure S4 Intracellular ROS and NOX2 are required for autophagy and proinflammatory responses in Mtb-Δeis-infected macrophages. (A) RAW 264.7 cells transfected with GFP-LC3 cDNA were infected with Mtb-Δeis (MOI = 10) in the presence or absence of DPI (10 µM), NAC (20 mM), catalase (Cat, 1 mU/mL), or tiron (5 mM). Formation of GFP-LC3 vacuoles (dots) was determined in transfected cells, and at least 250 cells per well were counted. Left: representative immunofluorescence images; right: percentage of LC3-punctated cells. (B) BMDMs from WT and NOX2-KO mice were infected with Mtb-WT, Mtb-Δeis, or Mtb-c-eis (MOI = 10). After 30 min, ROS production (DHE staining) was determined by flow cytometry (left). Quantitative analysis of ROS generation in WT- and NOX2-deficient BMDMs (right). Data represent the mean±SD of three independent experiments. (C) BMDMs from WT and NOX2 KO mice were treated with rapamycin (Rapa; 20 µg/mL) or staurosporine (STS; 500 nM), or nutrient-starved (Starv; maintained in HBSS) for 8 h. Numbers of LC3-punctated cells (counted manually) are shown. Data are presented as the mean±SD of at least three separate experiments, each performed in triplicate. (D) BMDMs from WT and NOX2 KO mice were infected with Mtb-WT, Mtb-Δeis, or Mtb-c-eis for 6 h and then subjected to RT-PCR analysis. A gel representative of three independent replicates is shown. *** p<0.001, vs. SC (A); WT mice (B). UI, uninfected; SC, solvent control (0.1% DMSO). (0.38 MB TIF) Click here for additional data file. Figure S5 Enhanced cell death in Mtb-Δeis-infected macrophages is regulated by autophagic pathways. (A) BMDMs were infected with Mtb-WT, Mtb-Δeis, or Mtb-c-eis at the indicated MOIs for 4 h, washed to remove unbound mycobacteria, and then incubated in complete DMEM at 37°C in 5% CO2 for the indicated periods of time. Cells were stained with PI and then examined by fluorescence microscopy. (B) Cell death was determined in RAW 264.7 cells transfected with specific siRNA for beclin-1, atg5, or non-specific scrambled siRNA (siNS) before infection with Mtb-WT, Mtb-Δeis, or Mtb-c-eis, as described in the Materials and Methods. After 36 h, cells were stained with PI and examined by fluorescence microscopy, as described in the Materials and Methods. Transfection efficiency was assessed by RT-PCR (inset). (C) Experimental conditions were identical to those outlined in panel A. Cell viability was assessed by trypan blue staining. Data are presented as the mean±SD of three separate experiments, each performed in duplicate. *p<0.05, ***p<0.001, vs. Mtb-WT-infected condition (A). (0.16 MB TIF) Click here for additional data file.