- Record: found
- Abstract: found
- Article: found
Transcriptome analysis of the effect of C-C chemokine receptor 5 deficiency on cell response to Toxoplasma gondii in brain cells
Read this article at
Abstract
Background
Infection with Toxoplasma gondii is thought to damage the brain and be a risk factor for neurological and psychotic disorders. The immune response-participating chemokine system has recently been considered vital for brain cell signaling and neural functioning. Here, we investigated the effect of the deficiency of C-C chemokine receptor 5 (CCR5), which is previously reported to be associated with T. gondii infection, on gene expression in the brain during T. gondii infection and the relationship between CCR5 and the inflammatory response against T. gondii infection in the brain.
Results
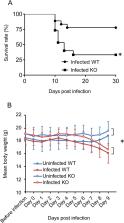
We performed a genome-wide comprehensive analysis of brain cells from wild-type and CCR5-deficient mice. Mouse primary brain cells infected with T. gondii were subjected to RNA sequencing. The expression levels of some genes, especially in astrocytes and microglia, were altered by CCR5-deficiency during T. gondii infection, and the gene ontology and Kyoto Encyclopedia of Genes and Genomes analysis revealed an enhanced immune response in the brain cells. The expression levels of genes which were highly differentially expressed in vitro were also investigated in the mouse brains during the T. gondii infections. Among the genes tested, only Saa3 (serum amyloid A3) showed partly CCR5-dependent upregulation during the acute infection phase. However, analysis of the subacute phase showed that in addition to Saa3, Hmox1 may also contribute to the protection and/or pathology partly via the CCR5 pathway.
Conclusions
Our results indicate that CCR5 is involved in T. gondii infection in the brain where it contributes to inflammatory responses and parasite elimination. We suggest that the inflammatory response by glial cells through CCR5 might be associated with neurological injury during T. gondii infection to some extent.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis.
- Record: found
- Abstract: found
- Article: not found
The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase.
- Record: found
- Abstract: found
- Article: not found