- Record: found
- Abstract: found
- Article: found
Involvement of the Bradykinin B 1 Receptor in Microglial Activation: In Vitro and In Vivo Studies
Read this article at
Abstract
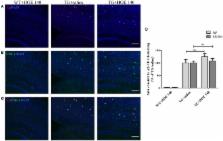
The importance of brain inflammation to Alzheimer’s disease (AD) pathogenesis has been accepted of late, with it currently being held that brain inflammation aggravates AD pathology. One important aspect of brain inflammation is the recruitment and activation of microglia, a process termed microgliosis. Kinins and bradykinin (BK), in particular, are major pro-inflammatory mediators in the periphery, although all of the factors comprising the kinin system have also been described in the brain. Moreover, it was shown that the amyloid β (Aβ) peptide (a component of AD plaques) enhances kinin secretion and activates BK receptors that can, in turn, stimulate Aβ production. Still, the role of bradykinin in modulating brain inflammation and AD is not completely understood. In this study, we aimed to investigate the roles of the bradykinin B 1 receptor (B 1R) and bradykinin B 2 receptor (B 2R) in regulating microglial secretion of pro-inflammatory factors in vitro. Furthermore, the effects of intranasal administration of specific B 1R and B 2R antagonists on Aβ burden and microglial accumulation in the brains of transgenic AD mice were studied. The data obtained show that neither R-715 (a B 1R antagonist) nor HOE 140 (a B 2R antagonist) altered microglial cell viability. However, R-715, but not HOE 140, markedly increased lipopolysaccharide-induced nitric oxide (NO) and tumor necrosis factor-alpha (TNF-α) release, as well as inducible nitric oxide synthase expression in BV2 microglial cells. Neither antagonist altered NO nor TNF-α production in non-stimulated cells. We also showed that intranasal administration of R-715 but not HOE 140 to 8-week-old 5X familial AD mice enhanced amyloid burden and microglia/macrophage accumulation in the cortex. To conclude, we provide evidence supporting a role of B 1R in brain inflammation and in the regulation of amyloid deposition in AD mice, possibly with microglial/macrophage involvement. Further studies are required to test whether modulation of this receptor can serve as a novel therapeutic strategy for AD.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Microglial cells internalize aggregates of the Alzheimer's disease amyloid beta-protein via a scavenger receptor.
- Record: found
- Abstract: found
- Article: not found
Intranasal Insulin as a Treatment for Alzheimer’s Disease: A Review of Basic Research and Clinical Evidence
- Record: found
- Abstract: found
- Article: not found