- Record: found
- Abstract: found
- Article: found
Polyproline as a Minimal Antifreeze Protein Mimic That Enhances the Cryopreservation of Cell Monolayers
Read this article at
Abstract
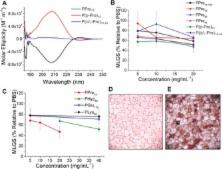
Tissue engineering, gene therapy, drug screening, and emerging regenerative medicine therapies are fundamentally reliant on high‐quality adherent cell culture, but current methods to cryopreserve cells in this format can give low cell yields and require large volumes of solvent “antifreezes”. Herein, we report polyproline as a minimum (bio)synthetic mimic of antifreeze proteins that is accessible by solution, solid‐phase, and recombinant methods. We demonstrate that polyproline has ice recrystallisation inhibition activity linked to its amphipathic helix and that it enhances the DMSO cryopreservation of adherent cell lines. Polyproline may be a versatile additive in the emerging field of macromolecular cryoprotectants.
Related collections
Most cited references53
- Record: found
- Abstract: not found
- Article: not found
Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide
- Record: found
- Abstract: not found
- Article: not found