- Record: found
- Abstract: found
- Article: found
Biotransformation of Methoxyflavones by Selected Entomopathogenic Filamentous Fungi
Read this article at
Abstract
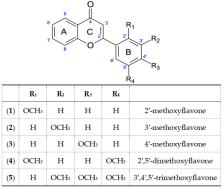
The synthesis and biotransformation of five flavones containing methoxy substituents in the B ring: 2′-, 3′-, 4′-methoxyflavones, 2′,5′-dimethoxyflavone and 3′,4′,5′-trimethoxyflavone are described. Strains of entomopathogenic filamentous fungi were used as biocatalysts. Five strains of the species Beauveria bassiana (KCh J1.5, J2.1, J3.2, J1, BBT), two of the species Beauveria caledonica (KCh J3.3, J3.4), one of Isaria fumosorosea (KCh J2) and one of Isaria farinosa (KCh KW 1.1) were investigated. Both the number and the place of attachment of the methoxy groups in the flavonoid structure influenced the biotransformation rate and the amount of nascent products. Based on the structures of products and semi-products, it can be concluded that their formation is the result of a cascading process. As a result of enzymes produced in the cells of the tested strains, the test compounds undergo progressive demethylation and/or hydroxylation and 4- O-methylglucosylation. Thirteen novel flavonoid 4- O-methylglucosides and five hydroxy flavones were isolated and identified.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Microbial biotransformation of bioactive flavonoids.
- Record: found
- Abstract: found
- Article: not found
Enzymatically modified isoquercitrin, alpha-oligoglucosyl quercetin 3-O-glucoside, is absorbed more easily than other quercetin glycosides or aglycone after oral administration in rats.

- Record: found
- Abstract: found
- Article: found