- Record: found
- Abstract: found
- Article: found
Impact of Age-Dependent Adventitia Inflammation on Structural Alteration of Abdominal Aorta in Hyperlipidemic Mice
Read this article at
Abstract
Background
The adventitia is suggested to contribute to vascular remodeling; however, the site-selective inflammatory responses in association with the development of atherosclerosis remain to be elucidated.
Methods and Results
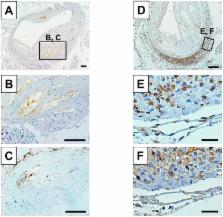
Wild-type or apolipoprotein E knockout male C57BL/6J background mice were fed standard chow for 16, 32, and 52 weeks, and the morphology of the aortic arch, descending aorta, and abdominal aorta was compared. Atheromatous plaque formation progressed with age, particularly in the aortic arch and abdominal aorta but not in the descending aorta. In addition, we found that the numbers of macrophages, T-lymphocytes, and microvessels, assessed by anti-F4/80, CD3, and CD31 antibodies, were higher in the adventitia of the abdominal aorta at 52 weeks. These numbers were positively correlated with plaque formation, but negatively correlated with elastin content, resulting in the enlargement of the total vessel area. In aortic tissues, interleukin-6 levels increased in the atheromatous plaque with age, whereas the level of regulated on activation, normal T cell expressed and secreted (RANTES) increased with age, and compared with other sites, it was particularly distributed in inflammatory cells in the adventitia of the abdominal aorta.
Related collections
Most cited references27
- Record: found
- Abstract: not found
- Article: not found
Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a "set up" for vascular disease.
- Record: found
- Abstract: found
- Article: not found
Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques.
- Record: found
- Abstract: found
- Article: not found