- Record: found
- Abstract: found
- Article: found
Use of Skin Testing Screening and Desensitization Before the First Exposure of Rituximab
Read this article at
Abstract
Objective
This study aimed to evaluate the incidence and severity of immediate hypersensitivity reactions (HSRs) in the first exposure to rituximab with the adoption of skin testing screening and desensitization and investigate the value of skin testing as a predictive tool for immediate HSR to rituximab.
Methods
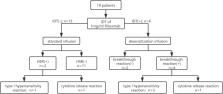
This was a prospective intervention study. Patients with hematological malignancies who required rituximab were recruited. Skin testing screening with rituximab was conducted before the first infusion. Patients with positive skin testing results underwent desensitization, while those with negative results received rituximab at a standard infusion rate. All immediate HSRs were recorded, and the predictive value of positive skin testing results for immediate HSRs to rituximab was analyzed.
Results
In the 19 patients who adopted the novel protocol, six patients (31.6%) had immediate HSRs during the first infusion, with three mild reactions (15.8%), two moderate reactions (10.5%), and only one severe reaction (5.3%). The positive predictive value of intradermal test (IDT) with 1 mg/mL rituximab solution for immediate HSR was 100%, and the negative predictive value was 84.6%.
Related collections
Most cited references17
- Record: found
- Abstract: found
- Article: not found
Clinical features and severity grading of anaphylaxis.
- Record: found
- Abstract: found
- Article: not found
Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose.
- Record: found
- Abstract: found
- Article: not found