- Record: found
- Abstract: found
- Article: found
Addiction and the adrenal cortex
Abstract
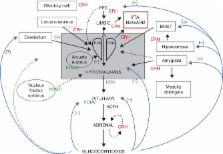
Substantial evidence shows that the hypophyseal–pituitary–adrenal (HPA) axis and corticosteroids are involved in the process of addiction to a variety of agents, and the adrenal cortex has a key role. In general, plasma concentrations of cortisol (or corticosterone in rats or mice) increase on drug withdrawal in a manner that suggests correlation with the behavioural and symptomatic sequelae both in man and in experimental animals. Corticosteroid levels fall back to normal values in resumption of drug intake. The possible interactions between brain corticotrophin releasing hormone (CRH) and proopiomelanocortin (POMC) products and the systemic HPA, and additionally with the local CRH–POMC system in the adrenal gland itself, are complex. Nevertheless, the evidence increasingly suggests that all may be interlinked and that CRH in the brain and brain POMC products interact with the blood-borne HPA directly or indirectly. Corticosteroids themselves are known to affect mood profoundly and may themselves be addictive. Additionally, there is a heightened susceptibility for addicted subjects to relapse in conditions that are associated with change in HPA activity, such as in stress, or at different times of the day. Recent studies give compelling evidence that a significant part of the array of addictive symptoms is directly attributable to the secretory activity of the adrenal cortex and the actions of corticosteroids. Additionally, sex differences in addiction may also be attributable to adrenocortical function: in humans, males may be protected through higher secretion of DHEA (and DHEAS), and in rats, females may be more susceptible because of higher corticosterone secretion.
Related collections
Most cited references192
- Record: found
- Abstract: found
- Article: not found
Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety.
- Record: found
- Abstract: found
- Article: not found
Anatomy and regulation of the central melanocortin system.
- Record: found
- Abstract: found
- Article: not found