- Record: found
- Abstract: found
- Article: found
miR-378 Activates the Pyruvate-PEP Futile Cycle and Enhances Lipolysis to Ameliorate Obesity in Mice
Read this article at
Abstract
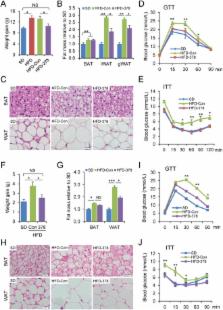
Obesity has been linked to many health problems, such as diabetes. However, there is no drug that effectively treats obesity. Here, we reveal that miR-378 transgenic mice display reduced fat mass, enhanced lipolysis, and increased energy expenditure. Notably, administering AgomiR-378 prevents and ameliorates obesity in mice. We also found that the energy deficiency seen in miR-378 transgenic mice was due to impaired glucose metabolism. This impairment was caused by an activated pyruvate-PEP futile cycle via the miR-378-Akt1-FoxO1-PEPCK pathway in skeletal muscle and enhanced lipolysis in adipose tissues mediated by miR-378-SCD1. Our findings demonstrate that activating the pyruvate-PEP futile cycle in skeletal muscle is the primary cause of elevated lipolysis in adipose tissues of miR-378 transgenic mice, and it helps orchestrate the crosstalk between muscle and fat to control energy homeostasis in mice. Thus, miR-378 may serve as a promising agent for preventing and treating obesity in humans.
Graphical abstract
Highlights
-
•
Systemically administering AgomiR-378 prevents and ameliorates obesity in mice
-
•
miR-378 transgenic mice show energy deficiency due to impaired glucose metabolism
-
•
The impairment is caused by miR-378-activated pyruvate-PEP futile cycle in muscle
-
•
miR-378 governs energy homeostasis by increased lipolysis via targeting Scd1 in fat
Futile cycles are generally regarded as energetically wasteful processes that are avoided in metabolic pathways. Zhang et al. demonstrate miR-378-activated pyruvate-phosphoenolpyruvate futile cycle plays a regulatory benefit for the energy homeostasis by orchestrating inter-organ crosstalk between skeletal muscle and fat in mice, resulting its implication in preventing and treating obesity.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss.
- Record: found
- Abstract: found
- Article: not found
Control of mitochondrial metabolism and systemic energy homeostasis by microRNAs 378 and 378*.
- Record: found
- Abstract: found
- Article: not found
