- Record: found
- Abstract: found
- Article: found
Direct transcatheter aortic valve implantation – one-year outcome of a case control study
Read this article at
Abstract
Introduction
Transaortic valve implantation (TAVI) has a well-established position in the treatment of high-risk and inoperable patients with severe aortic stenosis (AS). The TAVI protocol requires a pre-dilatation for native valve preparation.
Aim
To assess the safety and feasibility of TAVI without pre-dilatation and to compare it with the procedure with pre-dilatation.
Material and methods
Out of 101 TAVI patients, in 10 the procedure was performed without balloon predilatation, and 8 patients were included in the analysis. The procedural, echocardiographic, and clinical outcomes were compared with a case control matched cohort (1: 2 ratio). A 12-month follow-up was done in all cases.
Results
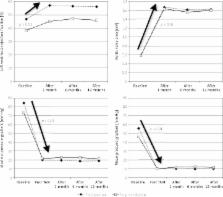
The procedure was successfully completed in all patients in the study group (SG), but there was one procedural failure in the control group (CG). All patients received a CoreValve (Medtronic) bioprosthesis. There was a significant immediate decrease in transvalvular gradients (TG) in both study arms after the procedure (SG: mean TG: from 46.0 ±14.0 mm Hg to 10.0 ±4.8 mm Hg, p < 0.001; CG: mean TG: from 55.9 ±12.0 mm Hg to 9.9 ±2.9 mm Hg, p < 0.001). A marked increase in the effective orifice areas was observed in both cohorts (SG: 1.63 ±0.13 cm 2 and CG: 1.67 ±0.25 cm 2, p = 0.75). The periprocedural complication rate was equally distributed in both arms. The 12-month all-cause mortality was 12.5% in both groups.
Related collections
Most cited references15
- Record: found
- Abstract: found
- Article: not found
Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document.
- Record: found
- Abstract: found
- Article: not found
Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study.
- Record: found
- Abstract: found
- Article: not found