- Record: found
- Abstract: found
- Article: not found
Divergence of multimodular polyketide synthases revealed by a didomain structure
Abstract
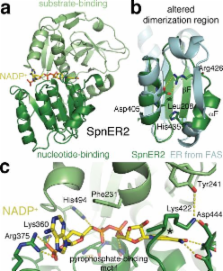
The enoylreductase (ER) is the final common enzyme from modular polyketide synthases (PKSs) to be structurally characterized. The 3.0 Å resolution structure of the didomain comprised of the ketoreductase (KR) and ER from the second module of the spinosyn PKS reveals that ER shares an ~600 Å 2 interface with KR distinct from that of the related mammalian fatty acid synthase (FAS). In contrast to the ER domains of the mammalian FAS, the ER domains of the second module of the spinosyn PKS do not make contact across the twofold axis of the synthase. This monomeric organization may have been necessary in the evolution of multimodular PKSs to enable acyl carrier proteins (ACPs) to access each of their cognate enzymes. The isolated ER domain showed activity towards a substrate analog, enabling the contributions of its active site residues to be determined.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
A graphical user interface to the CCP4 program suite.
- Record: found
- Abstract: found
- Article: not found
The crystal structure of a mammalian fatty acid synthase.
- Record: found
- Abstract: found
- Article: not found
