- Record: found
- Abstract: found
- Article: found
Identification of Potential Key Genes and Pathways in Enzalutamide-Resistant Prostate Cancer Cell Lines: A Bioinformatics Analysis with Data from the Gene Expression Omnibus (GEO) Database

Read this article at
Abstract
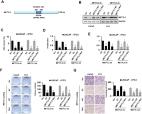
Enzalutamide (ENZ) has been approved for the treatment of advanced prostate cancer (PCa), but some patients develop ENZ resistance initially or after long-term administration. Although a few key genes have been discovered by previous efforts, the complete mechanisms of ENZ resistance remain unsolved. To further identify more potential key genes and pathways in the development of ENZ resistance, we employed the GSE104935 dataset, including 5 ENZ-resistant (ENZ-R) and 5 ENZ-sensitive (ENZ-S) PCa cell lines, from the Gene Expression Omnibus (GEO) database. Integrated bioinformatics analyses were conducted, such as analysis of differentially expressed genes (DEGs), Gene Ontology (GO) enrichment analysis, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis, protein-protein interaction (PPI) analysis, gene set enrichment analysis (GSEA), and survival analysis. From these, we identified 201 DEGs (93 upregulated and 108 downregulated) and 12 hub genes (AR, ACKR3, GPER1, CCR7, NMU, NDRG1, FKBP5, NKX3-1, GAL, LPAR3, F2RL1, and PTGFR) that are potentially associated with ENZ resistance. One upregulated pathway (hedgehog pathway) and seven downregulated pathways (pathways related to androgen response, p53, estrogen response, TNF- α, TGF- β, complement, and pancreas β cells) were identified as potential key pathways involved in the occurrence of ENZ resistance. Our findings may contribute to further understanding the molecular mechanisms of ENZ resistance and provide some clues for the prevention and treatment of ENZ resistance.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study.
- Record: found
- Abstract: found
- Article: not found
Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer.
- Record: found
- Abstract: found
- Article: found