- Record: found
- Abstract: found
- Article: found
Early intervention at home in infants with congenital brain lesion with CareToy revised: a RCT protocol
Read this article at
Abstract
Background
Congenital brain lesions expose infants to be at high-risk for being affected by neurodevelopmental disorders such as cerebral palsy (CP). Early interventions programs can significantly impact and improve their neurodevelopment. Recently, in the framework of the European CareToy (CT) Project ( www.caretoy.eu), a new medical device has been created to deliver an early, intensive, customized, intervention program, carried out at home by parents but remotely managed by expert and trained clinicians. Reviewing results of previous studies on preterm infants without congenital brain lesion, the CT platform has been revised and a new system created (CT-R).
This study describes the protocol of a randomised controlled trial (RCT) aimed to evaluate, in a sample of infants at high-risk for CP, the efficacy of CT-R intervention compared to the Infant Massage (IM) intervention.
Methods/design
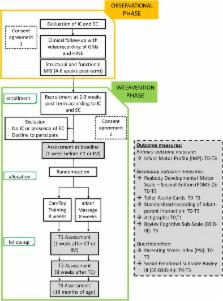
This RCT will be multi-centre, paired and evaluator-blinded. Eligible subjects will be preterm or full-term infants with brain lesions, in first year of age with predefined specific gross motor abilities. Recruited infants will be randomized into CT-R and IM groups at baseline (T0). Based on allocation, infants will perform an 8-week programme of personalized CareToy activities or Infant Massage. The primary outcome measure will be the Infant Motor Profile. On the basis of power calculation, it will require a sample size of 42 infants. Moreover, Peabody Developmental Motor Scales-Second Edition, Teller Acuity Cards, standardized video-recordings of parent-infant interaction and wearable sensors (Actigraphs) will be included as secondary outcome measures. Finally, parents will fill out questionnaires (Bayley Social-Emotional, Parents Stress Index). All outcome measures will be carried out at the beginning (T0) and at end of 8-weeks intervention period, primary endpoint (T1). Primary outcome and some secondary outcomes will be carried out also after 2 months from T1 and at 18 months of age (T2 and T3, respectively). The Bayley Cognitive subscale will be used as additional assessment at T3.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials.
- Record: found
- Abstract: found
- Article: not found
Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment.
- Record: found
- Abstract: found
- Article: not found