- Record: found
- Abstract: found
- Article: found
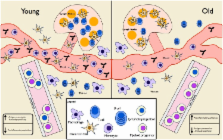
Immunosenescence and Novel Vaccination Strategies for the Elderly
Read this article at
Abstract
Vaccination remains the most effective prophylactic intervention for infectious disease in the healthcare professional’s toolkit. However, the efficacy and effectiveness of vaccines decrease with age. This becomes most apparent after an individual reaches 65–70 years old, and results from complex changes in the immune system that occur during aging. As such, new vaccine formulations and strategies that can accommodate age-related changes in immunity are required to protect this expanding population. Here, we summarize the consequences of immunosenescence on vaccination and how novel vaccination strategies can be designed to accommodate the aging immune system. We conclude that current vaccination protocols are not sufficient to protect our aging population and, in some cases, are an inefficient use of healthcare resources. However, researchers and clinicians are developing novel vaccination strategies that include modifying who and when we vaccinate and capitalize on existing vaccines, in addition to formulating new vaccines specifically tailored to the elderly in order to remedy this deficiency.
Related collections
Most cited references71
- Record: found
- Abstract: found
- Article: not found
Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review.
- Record: found
- Abstract: found
- Article: not found
Use of defined TLR ligands as adjuvants within human vaccines.
- Record: found
- Abstract: found
- Article: not found