- Record: found
- Abstract: found
- Article: found
Pharmacokinetics, pharmacodynamics and safety of CKD-519, a CETP inhibitor, in healthy subjects
Abstract
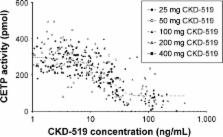
CKD-519 is a selective and potent cholesteryl ester transfer protein (CETP) inhibitor being developed for the treatment of dyslipidemia to raise high-density lipoprotein cholesterol. We investigated the safety, tolerability, pharmacokinetics, and pharmacodynamics of single doses of CKD-519 in healthy adult subjects. A randomized, double-blinded, placebo-controlled, single ascending dose study was performed. Eight healthy subjects were enrolled in each CKD-519 dose group (25, 50, 100, 200, or 400 mg) and randomized to CKD-519 (n=6) or matching placebo (n=2). CKD-519 reached the maximum plasma concentration (C max) at 5–6 h post-dose, and had a long terminal half-life ranging between 40–70 h. The area under the plasma concentration–time curve (AUC) and C max increased with the dose, however, C max and AUC normalized by dose decreased with each incremental dose. CETP activity decreased with dose, and the maximum decrease (63%–83%) was observed at 6–8 h post-dose. A sigmoid E max model best described the relationship between CKD-519 plasma concentrations and CETP activity with an EC 50 of 17.3 ng/mL. Overall, 11 adverse events (AEs) were observed. All AEs were mild or moderate in intensity, and resolved without any complications. There were no clinically significant effects on blood pressure. In conclusion, single doses of CKD-519 up to 400 mg were well tolerated and showed potent inhibition of CETP activity.
Most cited references16
- Record: found
- Abstract: found
- Article: not found
Cardiovascular disease epidemiology in Asia: an overview.
- Record: found
- Abstract: found
- Article: not found