- Record: found
- Abstract: found
- Article: found
Adverse drug reactions caused by drug-drug interactions reported to Croatian Agency for Medicinal Products and Medical Devices: a retrospective observational study
Read this article at
Abstract
Aim
To analyze potential and actual drug-drug interactions reported to the Spontaneous Reporting Database of the Croatian Agency for Medicinal Products and Medical Devices (HALMED) and determine their incidence.
Methods
In this retrospective observational study performed from March 2005 to December 2008, we detected potential and actual drug-drug interactions using interaction programs and analyzed them.
Results
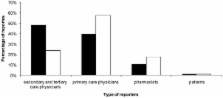
HALMED received 1209 reports involving at least two drugs. There were 468 (38.7%) reports on potential drug-drug interactions, 94 of which (7.8% of total reports) were actual drug-drug interactions. Among actual drug-drug interaction reports, the proportion of serious adverse drug reactions (53 out of 94) and the number of drugs (n = 4) was significantly higher ( P < 0.001) than among the remaining reports (580 out of 1982; n = 2, respectively). Actual drug-drug interactions most frequently involved nervous system agents (34.0%), and interactions caused by antiplatelet, anticoagulant, and non-steroidal anti-inflammatory drugs were in most cases serious. In only 12 out of 94 reports, actual drug-drug interactions were recognized by the reporter.
Conclusion
The study confirmed that the Spontaneous Reporting Database was a valuable resource for detecting actual drug-drug interactions. Also, it identified drugs leading to serious adverse drug reactions and deaths, thus indicating the areas which should be in the focus of health care education.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group.
- Record: found
- Abstract: found
- Article: not found
Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance.
- Record: found
- Abstract: found
- Article: not found