- Record: found
- Abstract: found
- Article: found
Toxicological assessment of polyhexamethylene biguanide for water treatment
Read this article at
Abstract
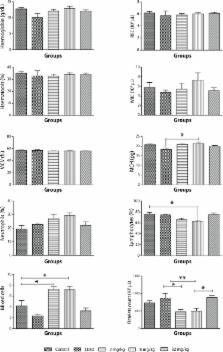
Polyhexamethylene biguanide (PHMB) is an antiseptic with antiviral and antibacterial properties used in a variety of products including wound care dressings, contact lens cleaning solutions, perioperative cleansing products, and swimming pool cleaners. There are regulatory concerns with regard to its safety in humans for water treatment. We decided to assess the safety of this chemical in Sprague-Dawley rats. PHMB was administered in a single dose by gavage via a stomach tube as per the manufacturer's instruction within a dose range of 2 mg/kg to 40 mg/kg. Subchronic toxicity studies were also conducted at doses of 2 mg/kg, 8 mg/kg and 32 mg/kg body weight and hematological, biochemical and histopathological findings of the major organs were assessed. Administration of a dose of 25.6 mg/kg, i.e. 1.6 mL of 0.4% PHMB solution (equivalent to 6.4x10 3 mg/L of 0.1% solution) resulted in 50% mortality. Histopathological analysis in the acute toxicity studies showed that no histopathological lesions were observed in the heart and kidney samples but 30% of the animals had mild hydropic changes in zone 1 of their liver samples, while at a dosage of 32 mg/kg in the subchronic toxicity studies, 50% of the animals showed either mild hepatocyte cytolysis with or without lymphocyte infiltration and feathery degeneration. Lymphocyte infiltration was, for the first time, observed in one heart sample, whereas one kidney sample showed mild tubular damage. The acute studies showed that the median lethal dose (LD 50) is 25.6 mg/kg (LC 50 of 1.6 mL of 0.4% PHMB. Subchronic toxicological studies also revealed few deleterious effects on the internal organs examined, as seen from the results of the biochemical parameters evaluated. These results have implications for the use of PHMB to make water potable.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Effect of bee venom on some blood and biochemical parameters in formaldehyde induced arthritis male rats in comparison with prednisolone drug
- Record: found
- Abstract: found
- Article: not found
Interaction of some polyhexamethylene biguanides and membrane phospholipids in Escherichia coli.
- Record: found
- Abstract: found
- Article: found