- Record: found
- Abstract: found
- Article: not found
The Thymus Is a Common Target Organ in Infectious Diseases
Read this article at
Abstract
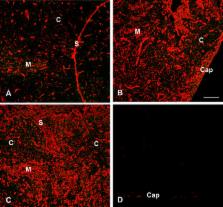
Infectious disease immunology has largely focused on the effector immune response, changes in the blood and peripheral lymphoid organs of infected individuals, and vaccine development. Studies of the thymus in infected individuals have been neglected, although this is progressively changing. The thymus is a primary lymphoid organ, able to generate mature T cells that eventually colonize secondary lymphoid organs, and is therefore essential for peripheral T cell renewal. Recent data show that normal thymocyte development and export can be altered as a result of an infectious disease. One common feature is the severe atrophy of the infected organ, mainly due to the apoptosis-related depletion of immature CD4 +CD8 + thymocytes. Additionally, thymocyte proliferation is frequently diminished. The microenvironmental compartment of the thymus is also affected, particularly in acute infectious diseases, with a densification of the epithelial network and an increase in the deposition of extracellular matrix. In the murine model of Chagas disease, intrathymic chemokine production is also enhanced, and thymocytes from Trypanosoma cruzi-infected mice exhibit greater numbers of cell migration-related receptors for chemokines and extracellular matrix, as well as increased migratory responses to the corresponding ligands. This profile is correlated with the appearance of potentially autoreactive thymus-derived immature CD4 +CD8 + T cells in peripheral organs of infected animals. A variety of infectious agents—including viruses, protozoa, and fungi—invade the thymus, raising the hypothesis of the generation of central immunological tolerance for at least some of the infectious agent-derived antigens. It seems clear that the thymus is targeted in a variety of infections, and that such targeting may have consequences on the behavior of peripheral T lymphocytes. In this context, thymus-centered immunotherapeutic approaches potentially represent a new tool for the treatment of severe infectious diseases.
Related collections
Most cited references97
- Record: found
- Abstract: found
- Article: not found
Lymphostromal interactions in thymic development and function.
- Record: found
- Abstract: found
- Article: not found
Molecular mechanisms governing thymocyte migration: combined role of chemokines and extracellular matrix.
- Record: found
- Abstract: found
- Article: not found
